Melting point for benzoic acid
Home » datasheet » Melting point for benzoic acidMelting point for benzoic acid
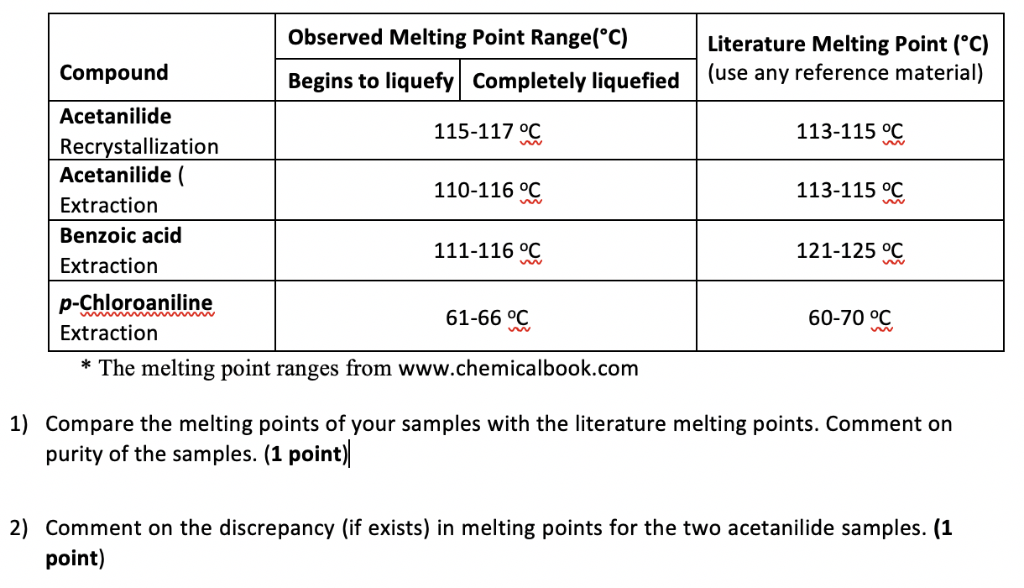
Melting Point For Benzoic Acid. Gain essential knowledge about the melting point technique. Weight of impure benzoic acid _____g. Measuring Melting Points - The melting point of a compound is the temperature at which the solid is in equilibrium with its liquid. What is the primary consideration in choosing a solvent for crystallizing.
 Pin On Crystallization From pinterest.co.uk
Pin On Crystallization From pinterest.co.uk
Use of apparatus and techniques. 249 C Literature LabNetwork old LN00195619 133 C 10 mmHg 2966803 C 760 mmHg FooDB FDB008739 249 C SynQuest 2621-1-21. At a temperature of 0 degrees celsius the solubility of benzoic acid in water corresponds to 17 grams per litre. It is obtained. You will compare the melting point of this impure sample to the benzoic acid recovered from recrystallization. Measuring Melting Points - The melting point of a compound is the temperature at which the solid is in equilibrium with its liquid.
122 C 252 F.
You will also find essential tips and hints for your daily work. When submitting the report the abstract should appear at the beginning of. 122 C 252 F. However the solubility of this compound in water increases when the temperature is increased as is the case with most compounds. 250 C 482 F. 158 C Jean-Claude Bradley Open Melting Point Dataset 16855 17199 28516 28517 28518.
 Source: chegg.com
Source: chegg.com
The purer the sample the narrower the melting point range. Definitions of the acid dissociation constant and pKa are given below the figures together with the definition of some classes of organic acids. Melting point of recrystallized benzoic acid _____ o C e. Use of apparatus and techniques. An unknown sample of benzoic acid 2-naphthol and naphthalene Table 1 was massed.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
4649C Flash point. Complete any post-lab questions. 34 gl 25C Boiling pointBoiling range. A sharp melting point is often evidence that a sample is fairly pure and a wide melting range is evidence that it is not pure. Use melting point apparatus.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Or they must have different arrangements of atoms or configurations. F benzoic acid 122-123 trans-stilbene 122-123 G cinnamic acid 1325-133 urea 1325-133 Results Discussion and Conclusion Write your results discussion of results and your conclusion. When your sample is dry measure the mass and calculate your percent recovery. 17 gL 0. CHEM 2423 Recrystallization of Benzoic Acid Dr.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
4-Aminobenzoic acid also known as para-aminobenzoic acid or PABA because the two functional groups are attached to the benzene ring across from one another in the para position is an organic compound with the formula H 2 NC 6 H 4 CO 2 H. An unknown sample of benzoic acid 2-naphthol and naphthalene Table 1 was massed. A sharp melting point is often evidence that a sample is fairly pure and a wide melting range is evidence that it is not pure. It is obtained. 250 C 482 F.
 Source: lgcstandards.com
Source: lgcstandards.com
CHEM 2423 Recrystallization of Benzoic Acid Dr. Benzoic acid is not very soluble in water. 249 C Alfa Aesar A14062 36230. 4649C Flash point. A monohydroxybenzoic acid that is benzoic acid with a hydroxy group at the ortho position.
 Source: odinity.com
Source: odinity.com
CHEM 2423 Recrystallization of Benzoic Acid Dr. Use of apparatus and techniques. 122 C 252 F. Use melting point apparatus. When your sample is dry measure the mass and calculate your percent recovery.

At a temperature of 0 degrees celsius the solubility of benzoic acid in water corresponds to 17 grams per litre. Vapour pressure. Definitions of the acid dissociation constant and pKa are given below the figures together with the definition of some classes of organic acids. The melting point range for pure solids is narrow usually only 1 to 2 degrees Celsius known as a sharp melting point. 523 K Solubility in water.
Source: chemspider.com
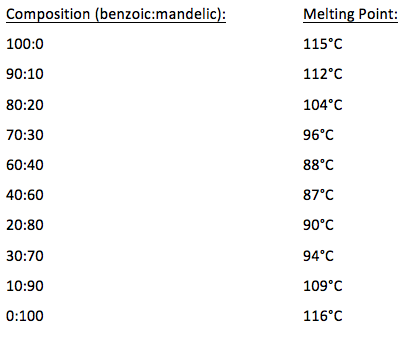
Weight of benzoic acid obtained after recrystallization Recovered x100 Weight of benzoic acid before recrystallization Note. It is obtained. 1224 deg C Solubilities. Melting point is also used for the identification and characterisation of a compound. The melting points of the recrystallized benzoic acid were 114- 122 C and 121-127 C both encompassing the expected value and allowing one to believe that the sample was close to pure which was the goal of recrystallization.
 Source: researchgate.net
Source: researchgate.net
Answer in space provided. Comment on the solubility of benzoic acid in water. It consists of a benzene ring substituted with amino. A monohydroxybenzoic acid that is benzoic acid with a hydroxy group at the ortho position. 81 Use of apparatus and techniques.
 Source: pinterest.co.uk
Source: pinterest.co.uk
2492C 760 mmHg Partition coefficient n-octanolwater. Weight of impure benzoic acid _____g. What is the primary consideration in choosing a solvent for crystallizing. When submitting the report the abstract should appear at the beginning of. 3000 C Boiling point.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title melting point for benzoic acid by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.