Melting point cadmium
Home » datasheet » Melting point cadmiumMelting point cadmium
Melting Point Cadmium. The melting point depends on the pressure. In the air cadmium is rapidly oxidized into cadmium oxide. The melting point or rarely liquefaction point of a solid is the temperature at which a sustance changes state from solid to liquid at atmospheric pressure. Value given for diamond form.
 Cadmium Uses Properties Facts Britannica From britannica.com
Cadmium Uses Properties Facts Britannica From britannica.com
Value given for monoclinic beta form. Melting point of Calcium. Value given for hexagonal gray form. Up to date curated data provided by Mathematicas ElementData. As a refractory metal generally the melting point is higher than 1650 with the highest melting point it has good high-temperature strength. Value given for alpha form.
In bulk however the metal is not that dangerous and can alloy in solders as a safer alternative to lead.
14025 K-258975 C-434 F. No decomposition if used according to specifications Temperatures over 85C short-circuit of electrode connections. Engineering ToolBox - Resources Tools and Basic Information for Engineering and Design of Technical Applications. Melting point is a characteristic property of solid crystalline substances. Tungsten takes the 4th spot in our list of the materials with the highest melting point. Not applicable Boiling point.
 Source: slideplayer.com
Source: slideplayer.com
The kitchenware made of pyrex will never melt unless heat exceeds this limit. Not applicable Flash point. As a refractory metal generally the melting point is higher than 1650 with the highest melting point it has good high-temperature strength. 2 - Atomic number-259. The melting point of pyrex is between 820C to 1250C.
 Source: physics.stackexchange.com
Source: physics.stackexchange.com
Product Melting Point o CBoiling Point o CAgate. It is used in research and development as well as in quality control in. Melting point of Cadmium. 767C 1413F 1040 K Block. Not applicable Boiling point.
 Source: cadmium.org
Source: cadmium.org
Cast Iron Gray 1175-1290. However when reactive gases or vapour such as carbon dioxide water vapour sulfur dioxide. Atomic number - Name alphabetically-272. A substances melting point depends on pressure and is usually specified at standard pressure in reference materials. Not applicable Boiling point.
 Source: researchgate.net
Source: researchgate.net
D Density g cm 3. Engineering ToolBox - Resources Tools and Basic Information for Engineering and Design of Technical Applications. The melting point is specific for a given substance. We say that such a body melts. Thus higher the stronger the bond between the atoms higher will be the melting point.
 Source:
Source:
At the melting point the two phases of a substance liquid and vapor have identical free energies and therefore are equally likely to exist. At the melting point the solid and liquid phases exist in equilibrium. Weight in Troy OzsCu In. Melting point of Calcium. However when reactive gases or vapour such as carbon dioxide water vapour sulfur dioxide.

At the melting point the solid and liquid phase exist in equilibrium. Melting point of Canola Oil -10 C. Melting point is a characteristic property of solid crystalline substances. Cast Iron Gray 1175-1290. Click on any elements name for further chemical properties environmental data or health effects.
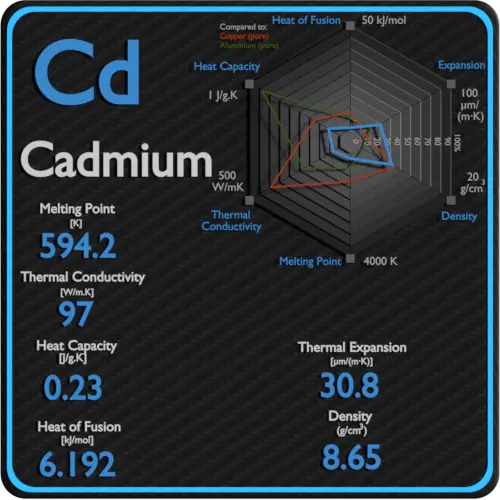 Source: material-properties.org
Source: material-properties.org
D Density g cm 3. Click on any elements name for further chemical properties environmental data or health effects. Engineering ToolBox - Resources Tools and Basic Information for Engineering and Design of Technical Applications. Value given for hexagonal gray form. In the following table the use row is the value recommended for use in other Wikipedia pages in order to maintain consistency across content.
 Source: britannica.com
Source: britannica.com
The melting point also defines a condition in which the solid and liquid can exist in equilibrium. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard pressure. Value given for monoclinic beta form. Value given for hexagonal gray form.
 Source: assignmentpoint.com
Source: assignmentpoint.com
The melting point or rarely liquefaction point of a solid is the temperature at which a sustance changes state from solid to liquid at atmospheric pressure. Metals and Alloys - Melting Temperatures Melting temperatures of common metals and alloys. The chemical elements of the periodic chart sorted by. Let us look at the elements in the ascending order of their melting points. Value given for yellow phosphorus form.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Which essentially implies breaking a few bonds. The melting point depends on the pressure. Helium does not solidify at standard pressure. This list contains the 118 elements of chemistry. Let us look at the elements in the ascending order of their melting points.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title melting point cadmium by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.