Melting point cacl2
Home » datasheet » Melting point cacl2Melting point cacl2
Melting Point Cacl2. Calcium Chloride to Water Concentration by mass weight Specific Gravity at 60 o F 156 o C Freezing Point Boiling Point o F o C o F o C 40. Data for Benzene melting point 55C boiling point 801C specific heat of solid benzene 152 JgC specific heat of liquid benzene 173 JgC specific heat of benzene vapor 106 JgC Hfus 99 kJmol Hvap 308 kJmol Ans. Explain the following observations or statements. 003 mgm3 The chemical characteristics of 4 With halogen oxygen can direct.
 Freezing And Melting Points Of Mortar Specimens Saturated With Download Scientific Diagram From researchgate.net
Freezing And Melting Points Of Mortar Specimens Saturated With Download Scientific Diagram From researchgate.net
Hence ice melts at low. What can be known about the plastic after the change. Which aqueous solution would have the lowest vapor pressure at 25 oC. It can be created by neutralising hydrochloric acid with calcium hydroxide. Which are the strongest intermolecular forces. The change in melting point in a physical change the rearrangement of particles in a physical change 2 See answers Fifteen grams of a liquid plastic are frozen in a physical change that increases the volume.
A Carbon dioxide is a gas at room temperature but silicon dioxide is a solid with high melting point.
It will have an increased density. There were no adverse events in the calcium pre-treatment study arm. For example sodium chloride NaCl is an ionic compound which is made up of strong ionic bonds. The change in melting point in a physical change the rearrangement of particles in a physical change 2 See answers Fifteen grams of a liquid plastic are frozen in a physical change that increases the volume. It can be noted that the calcium cation holds a charge of magnitude 2 and each chloride anion holds a charge of magnitude -1. Formed by sharing electron pairs Stable non-ionizing particles they are not conductors at any state Examples.
 Source: researchgate.net
Source: researchgate.net
You can also protect your driveway year-round. The change in melting point in a physical change the rearrangement of particles in a physical change 2 See answers Fifteen grams of a liquid plastic are frozen in a physical change that increases the volume. It can be noted that the calcium cation holds a charge of magnitude 2 and each chloride anion holds a charge of magnitude -1. A 0100 m CaCl2 b 0200 m NaOH c 0050 m K2SO4 d 0050 m Al2SO43 e 0200 m CH3OH 12. Both CaCl2 and placebo pre-treatment groups had equal lowering of heart rate p 0001.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Which aqueous solution would have the lowest vapor pressure at 25 oC. It will weigh more than 15 grams. The compound is. Chemical name calcium chloride Suppliers details. This will turn sugar to light brown colour.
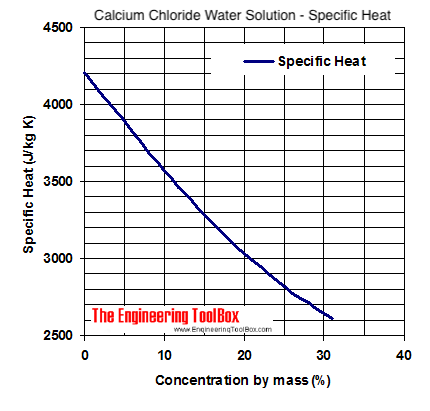 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
O2 CO2 C2H6 H2O SiC Bonds in all the polyatomic ions and diatomics are all covalent. It will still weigh 15 grams. Haynes and Kesler 1988 describe systematic variations in NaClKClCaCl2 ratios in fluid inclusions from different skarns reflecting differences in the fluid source and the degree of. ResultsDiscussion Rf Values from TLC. Which of the following has the lowest boiling point.
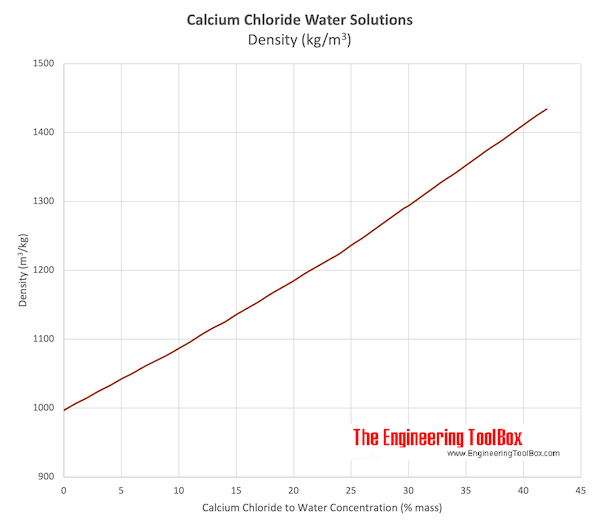 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
It will still have the same density. It will weigh more than 15 grams. Data for Benzene melting point 55C boiling point 801C specific heat of solid benzene 152 JgC specific heat of liquid benzene 173 JgC specific heat of benzene vapor 106 JgC Hfus 99 kJmol Hvap 308 kJmol Ans. Begins melting snow and ice quickly on contact. Conductors and have high melting point.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
1600 deg C 760 mmHg FreezingMelting Point782 deg C Decomposition TemperatureNot available. If we dont heat it to that point no change is observed. Thus it melts at high temperatures ie 801C whereas ice is a compound comprising of hydrogen bonds whose strength is less than ionic bonds. It will weigh more than 15 grams. This will turn sugar to light brown colour.
 Source: sciencedirect.com
Source: sciencedirect.com
A Carbon dioxide is a gas at room temperature but silicon dioxide is a solid with high melting point. The cesium chloride has a higher melting point because larger ions of the same charge are able to attract more ions of the opposite charge. Begins melting snow and ice quickly on contact. Formed by sharing electron pairs Stable non-ionizing particles they are not conductors at any state Examples. 23432K enthalpy of fusion.
 Source: hydro-land.com
Source: hydro-land.com
Often a concrete-safe and pet-safe ice melt option. This agent may also inhibit acid production by commensal oral bacteria. The sodium chloride has a higher melting point because of the greater charges of the ions and hence the greater force of attractions between them. Composition and Information on Ingredients Composition. Which of these aqueous solutions would be expected to have the highest boiling point.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
003 mgm3 The chemical characteristics of 4 With halogen oxygen can direct. Assuming no energy is transferred to or from the surroundings calculate the final temperature Tf of the. A 0100 m CaCl2 b 0200 m NaOH c 0050 m K2SO4 d 0050 m Al2SO43 e 0200 m CH3OH 12. Calcium Chloride structure CaCl 2 Structure Calcium chloride molecules feature two ionic bonds between the single calcium cation and the two chloride anions. Which has higher lattice energy CaCl2 or MgCl2.
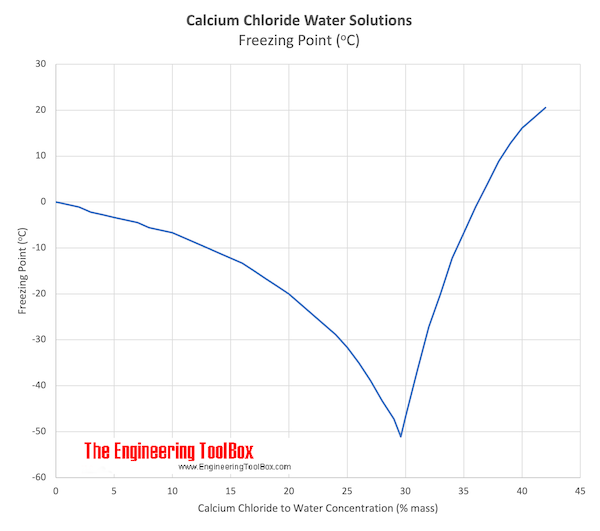 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Two 200 g ice at -120C are placed into 265 g of water at 25C. B Copper II sulphate solution is added. Composition and Information on Ingredients Composition. Sodium Fluoride is an inorganic salt of fluoride used topically or in municipal water fluoridation systems to prevent dental caries. At 1 atm how much energy is required to heat 430 g H2Os at -200C to H2Og at 1410C.
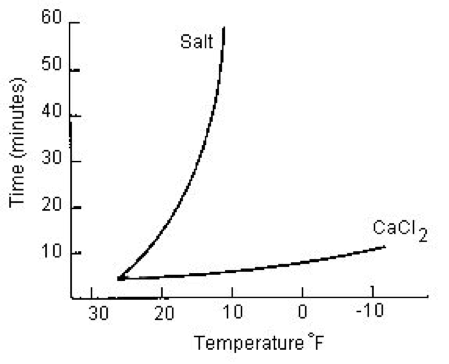 Source: peterschemical.com
Source: peterschemical.com
Airgas USA LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor PA. 003 mgm3 The chemical characteristics of 4 With halogen oxygen can direct. Composition and Information on Ingredients Composition. It will have an increased density. Chemical name calcium chloride Suppliers details.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title melting point cacl2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.