Melting point benzoic acid
Home » datasheet » Melting point benzoic acidMelting point benzoic acid
Melting Point Benzoic Acid. Melting point of recrystallized benzoic acid _____ o C e. The purpose of this experiment is to learn the technique of recrystallization by purifying benzoic acid. Determine the melting point ranges of the impure benzoic acid and the crystallized benzoic acid after it is dry. It consists of a benzene ring substituted with amino.
 Benzoic Acid 65 85 0 C7h6o2 Density Melting Point Boiling Point Structural Formula Synthesis From chemsynthesis.com
Benzoic Acid 65 85 0 C7h6o2 Density Melting Point Boiling Point Structural Formula Synthesis From chemsynthesis.com
Salicylic acid and sulphuric acid are both corrosive and a skin irritant at high concentration. Benzoic acid is a constituent of Whitfields ointment which is used for the treatment of fungal skin diseases such as tinea ringworm and athletes foot. PABA is a white solid although commercial samples can appear gray. 570C Evaporation rate. F benzoic acid 122-123 trans-stilbene 122-123 G cinnamic acid 1325-133 urea 1325-133 Results Discussion and Conclusion Write your results discussion of results and your conclusion. 3 Bring approximately 200 mL of water to a boil using the 250 mL round bottom flask fitted with a clamp as a.
At a temperature of 0 degrees celsius the solubility of benzoic acid in water corresponds to 17 grams per litre.
Benzoic acid is a constituent of Whitfields ointment which is used for the treatment of fungal skin diseases such as tinea ringworm and athletes foot. The purpose of this experiment is to learn the technique of recrystallization by purifying benzoic acid. Structural formula of the benzoic acid 7. Reserve a small sample of your benzoic acid. Benzoic Acid Created by Global Safety Management Inc. Scoop a small amount of the powder into the opening of the melting point tube and gently tap the tube on the benchtop to move the sample.
 Source: researchgate.net
Source: researchgate.net
Let the crystals dry in air. Take a melting point and assess its purity by comparing the measured melting point with the literature value. 3000 C Boiling point. Complete any post-lab questions. For example ice is a solid form of water that melts at 0 degrees Celsius32 degrees Fahrenheit and changes to its liquid form.
 Source: lgcstandards.com
Source: lgcstandards.com
Reserve a small sample of your benzoic acid. Gilbert and Martin Experimental. Then a small. Benzoic acid is not very soluble in cold water but it is soluble in hot water. Calculate the percent recovered using the following written formula and determine the melting point of your recrystallized benzoic acid.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
It is obtained from the bark of the white willow and wintergreen leaves. 249 C Food and Agriculture Organization of the United Nations Benzoic acid. 2-methylpropanoic acid isobutter C 3 H 7 COOH-56. 17 gL 0. Sample preparation process using METTLER TOLEDO melting point tools.
 Source: chemsynthesis.com
Source: chemsynthesis.com
158 C Jean-Claude Bradley Open Melting Point Dataset 16855 17199 28516 28517 28518. Salicylic acid and sulphuric acid are both corrosive and a skin irritant at high concentration. Melting point is the temperature at which a solid turns into a liquid. 250 C 482 F. A solid compound changes to a.

However the solubility of this compound in water increases when the temperature is increased as is the case with most compounds. The melting points of the recrystallized benzoic acid were 114- 122 C and 121-127 C both encompassing the expected value and allowing one to believe that the sample was close to pure which was the goal of recrystallization. 2-methylpropanoic acid isobutter C 3 H 7 COOH-56. Answer in space provided. Calculate the percent recovered using the following written formula and determine the melting point of your recrystallized benzoic acid.

Determine the melting point ranges of the impure benzoic acid and the crystallized benzoic acid after it is dry. CH 3 CH 2 CH 2 COOH-57. 3000 C Boiling point. Comment on the solubility of benzoic acid in water. DescriptionUses Melting Point ºC Vanillin 1102 Natural vanilla flavoring 81-82 Dibenzofuran 2111 Minor constituent of coal tar 81-83 Acetamide 3111 Solvent plasticizer stabilizer 79-81 Azelaic Acid 0011 Rancidification product of fats 106-107 containing oleic acids o-Toluic Acid 1011 Substituted benzoic acid 103-105.
 Source: fishersci.co.uk
Source: fishersci.co.uk
When your sample is dry measure the mass and calculate your percent recovery. Determine the melting point ranges of the impure benzoic acid and the crystallized benzoic acid after it is dry. Benzoic acid is not very soluble in cold water but it is soluble in hot water. The melting point of solid is defined as the temperature at which the solid exists in equilibrium with its liquid under an external pressure of one atmosphere. 249 C Alfa Aesar A14062 36230.
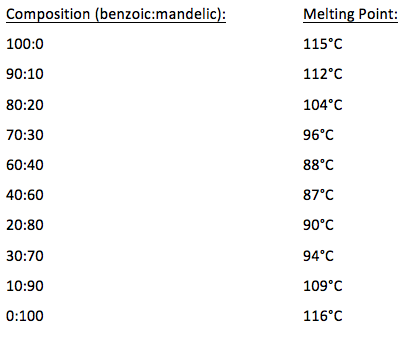 Source: odinity.com
Source: odinity.com
2 Weigh the impure benzoic acid crystals obtained last week using the analytical balance and place them in a 250 mL Erlenmeyer flask. Benzoic Acid Created by Global Safety Management Inc. When submitting the report the abstract should appear at the beginning of. Take a melting point and assess its purity by comparing the measured melting point with the literature value. CH 3 CH 2 COOH-207.
 Source: pinterest.co.uk
Source: pinterest.co.uk
570C Evaporation rate. When submitting the report the abstract should appear at the beginning of. Benzoic acid is not very soluble in water. Weight of benzoic acid obtained after recrystallization Recovered x100 Weight of benzoic acid before recrystallization Note. A solid compound changes to a.

3000 C Boiling point. Be sure to grind each sample well before introducing it into the melting point tube you may use a glass rod and a watch glass. Oxalic acid COOH 2. 3 Bring approximately 200 mL of water to a boil using the 250 mL round bottom flask fitted with a clamp as a. 570C Evaporation rate.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title melting point benzoic acid by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.