Melting point and solubility
Home » datasheet » Melting point and solubilityMelting point and solubility
Melting Point And Solubility. Our chief focus up to this point has been to discover and describe. As a pure material bronopol has a melting point of about 130 C. The four solvents water seems to be the best choice as a. Estimates aerobic and anaerobic biodegradability of organic chemicals using 7.
 Physical Properties Of Substances From breakingatom.com
Physical Properties Of Substances From breakingatom.com
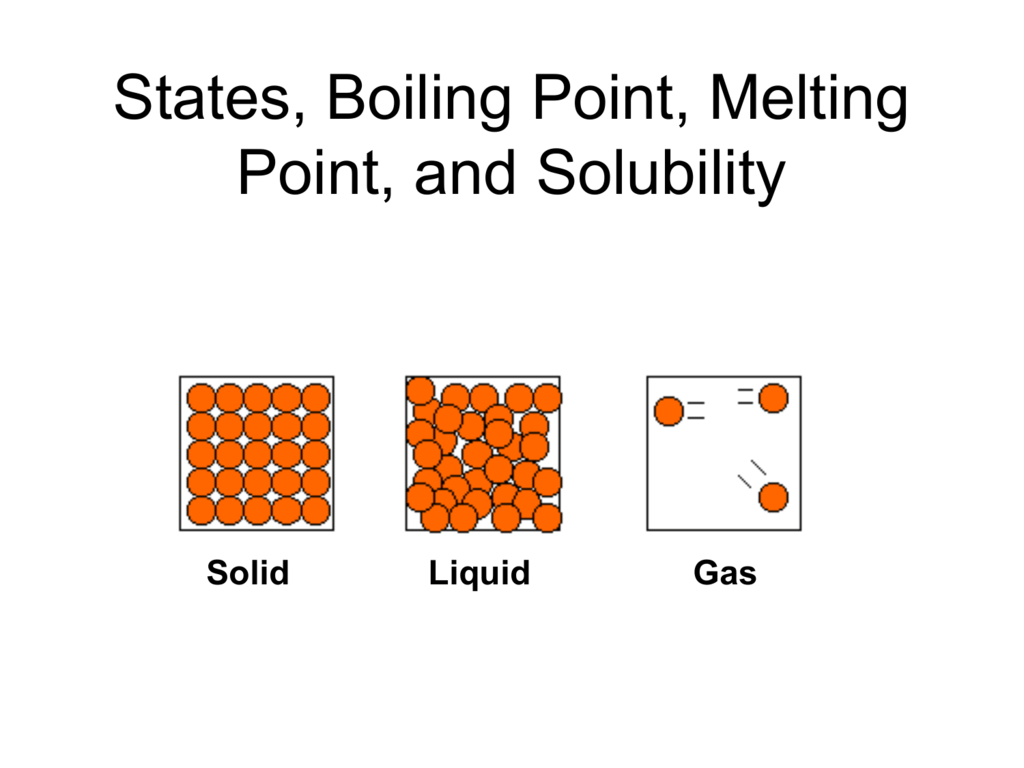
In the graphic on the left the solid and liquid. The three states of matter are. The presence of hydrogen bonding will lift the melting and boiling points. The molecule is the smallest observable group of uniquely bonded atoms that represent the composition configuration and characteristics of a pure compound. The dissolution process is achieved heating or changing the pH. Recrystallization of Benzoic Acid was performed according to the method of Gilbert and Morgan 2001 section 32.
Freezing point is the temperature at which a liquid changes to solid.
One of the. The size of the melting or boiling point will depend on the strength of the intermolecular forces. Solid liquid and gas. The molecule is the smallest observable group of uniquely bonded atoms that represent the composition configuration and characteristics of a pure compound. Recrystallization is a common method used to purify a sample. In the graphic on the left the solid and liquid.
 Source: breakingatom.com
Source: breakingatom.com
Once the solution had cooled and no crystals appeared to form 20mL of water was. Instead of using water to recrystallize benzoic acid ethanol was used in 32B part 1. Physical properties of matter include color hardness malleability solubility electrical conductivity density freezing points melting points and boiling points. It has been postulated that the lower the melting point of the permeant the greater is the solubility in a given solvent including the skin lipids Benson 2005. For full table with Density Liquid Denity at.
 Source: youtube.com
Source: youtube.com
Tritium appeared in the serum of both groups 30 min after the ip injection but the level was generally lower in the choline-deficient grouprats on the normal diet excreted more of the amino acid in the urine than did the choline-deficient rats in both groups. LBE is an eutectic. It is important in fate modeling. Sodium chloride NaCl is an ionic compound that consists of a multitude. Although the terms mass and weight are used almost interchangeably there is a difference between themMass is a measure of the quantity of matter which is constant all over the universeWeight is proportional to mass but depends on location in the universeWeight is the force exerted on a body by gravitational attraction usually by the earth.
 Source: researchgate.net
Source: researchgate.net
Physical properties of matter include color hardness malleability solubility electrical conductivity density freezing points melting points and boiling points. The presence of hydrogen bonding will lift the melting and boiling points. Flammable Gas Incompatibilities Reactivities Strong oxidizers bromine trifluoride chlorine trifluoride lithium Exposure Routes inhalation skin andor eye contact liquid Symptoms headache. Partition coefficients at 2224 C Solvent Combination Partition Co-efficient HexanolWater. Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodineUnder standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions I in a crystal latticeIt is used mainly as a nutritional supplement and in organic chemistryIt is produced industrially as the salt formed when.
 Source: researchgate.net
Source: researchgate.net
The three states of matter are. Partition coefficients at 2224 C Solvent Combination Partition Co-efficient HexanolWater. As a pure material bronopol has a melting point of about 130 C. DECOLORIZING A SOLUTION OF IMPURE ACETANILIDE AND HOT. Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodineUnder standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions I in a crystal latticeIt is used mainly as a nutritional supplement and in organic chemistryIt is produced industrially as the salt formed when.
 Source: researchgate.net
Source: researchgate.net
Solubility Test cold Solubility Test hot Water insoluble soluble pet ether insoluble insoluble ethanol fairly soluble readily soluble acetone very soluble readily soluble After looking at the solubility of impure acetanilide in. Natural mixed triglycerides have somewhat lower melting points the melting point of lard being near 30 º C whereas olive oil melts near -6 º C. Melting point - the temperature at which a solid turns into a liquid. As with boiling points the melting point of a solid is dependent on the strength of those attractive forces. LBE is an eutectic.
 Source: researchgate.net
Source: researchgate.net
At the melting point the heat added is used to break the attractive intermolecular forces of the solid instead of increasing kinetic energy and therefore the temperature remains constant. For full table with Density Liquid Denity at. Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodineUnder standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions I in a crystal latticeIt is used mainly as a nutritional supplement and in organic chemistryIt is produced industrially as the salt formed when. As the name implies a solid sample with suspect purity is dissolved into an appropriate solvent. It has been postulated that the lower the melting point of the permeant the greater is the solubility in a given solvent including the skin lipids Benson 2005.

As you know physical properties. After all the solid has melted once again the heat added goes to increasing the kinetic energy and temperature of the liquid molecules until the boiling point. As a pure material bronopol has a melting point of about 130 C. Freezing point is the temperature at which a liquid changes to solid. Recrystallization of Benzoic Acid was performed according to the method of Gilbert and Morgan 2001 section 32.
 Source: researchgate.net
Source: researchgate.net
In the graphic on the left the solid and liquid. LBE is an eutectic. One of the. At the boiling point once again the heat. Melting point of the solid.
 Source: studylib.net
Source: studylib.net
Freezing point is the temperature at which a liquid changes to solid. Since fats are valued over oils by some Northern European and North American populations vegetable oils are extensively converted to solid. Thus the melting points of triglycerides reflect their composition as shown by the following examples. The melting point and boiling point are related to changes of the state of matter. Melting point and freezing.
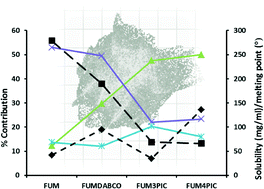
In two-phase systems bronopol partitions preferentially into the polar usually aqueous phase. In two-phase systems bronopol partitions preferentially into the polar usually aqueous phase. Flammable Gas Incompatibilities Reactivities Strong oxidizers bromine trifluoride chlorine trifluoride lithium Exposure Routes inhalation skin andor eye contact liquid Symptoms headache. The three states of matter are. Although the terms mass and weight are used almost interchangeably there is a difference between themMass is a measure of the quantity of matter which is constant all over the universeWeight is proportional to mass but depends on location in the universeWeight is the force exerted on a body by gravitational attraction usually by the earth.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title melting point and solubility by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.