Manganese dioxide boiling point
Home » datasheet » Manganese dioxide boiling pointManganese dioxide boiling point
Manganese Dioxide Boiling Point. Dioxide and oxidizes dissolved metals such as iron hydrogen sulfide and volatile organic chemicals VOCs. Manganese dioxide is used as the cathode material in dry-cell batteries. It is a hard metal. Chemical element Ti.
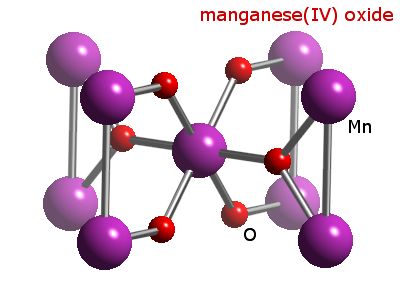 Webelements Periodic Table Manganese Manganese Dioxide From webelements.com
Webelements Periodic Table Manganese Manganese Dioxide From webelements.com
008 nm 2. It is a hard metal. Energy of first ionisation. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. Dioxide and oxidizes dissolved metals such as iron hydrogen sulfide and volatile organic chemicals VOCs. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds.
2334 K 2061 C 3742 F Density.
Energy of second ionisation. Page 2 of 4 Alkaline. Chemical element Ti. The main mining areas for manganese are in China Africa Australia and Gabon. The metal is obtained by reducing the oxide with sodium. Thorium is commonly found in monazite sands rare earth metals containing phosphate mineral.
 Source: webelements.com
Source: webelements.com
Carbon has been known about since ancient times. Standard potential - 105 V Mn 2 Mn Discovered. 2061C 3742F 2334 K Block. Titanium has a melting point of 1660 - 10C boiling point of 3287C specific gravity of 454 with a valence of 2 3 or 4. 008 nm 2.

Melting point - the temperature at which a solid turns into a liquid. They give the temperature at which the vapor pressure of the liquid is equal to atmospheric pressure at sea. Manganese is a pinkinsh-gray chemically active element. 009 nm 2. Titanium is only ductile when it is.
Energy of fourth ionisation. Pure titanium is a lustrous white metal with low density high strength and high corrosion resistance. It forms more compounds than any other element and forms the basis to all plant and animal life. First used as a solvent for uric acid the use of piperazine as an anthelmintic agent was first introduced in 1953Upon entry into the systemic circulation the drug is partly oxidized and partly eliminated as an unchanged compound. Monoclinic Coordination geometry.
 Source: americanelements.com
Source: americanelements.com
The process takes advantage of the fact that when a compressed gas is allowed to expand it cools. 0046 nm 7 Isotopes. Carbon is the fourth most abundant element in the universe by mass and the. ManganateVI salts can be produced by dissolving Mn compounds such as manganese dioxide in molten alkali while exposed to air. Energy of third ionisation.

For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. Thorium is a naturally-occurring element and it is estimated to be about three times more abundant than uranium. Energy of first ionisation. By blocking the pores the cuticle helps to preserve freshness and prevent microbial. For example water boils at 100C 212F at sea level but at 934C 2001.
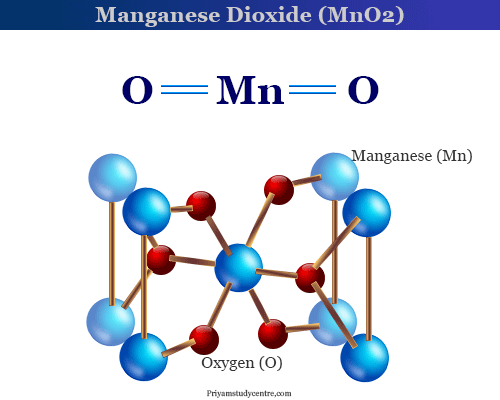 Source: priyamstudycentre.com
Source: priyamstudycentre.com
2334 K 2061 C 3742 F Density. The most important manganese compound is manganese dioxide in which manganese is in the 4 oxidation state and the black mineral pyrolusite is the chief source of manganese and all of its compounds. Related compounds Related. It is a hard metal. Energy of second ionisation.
 Source: chemicalbook.com
Source: chemicalbook.com
A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. Thorium is commonly found in monazite sands rare earth metals containing phosphate mineral. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Of the main components of air oxygen has the highest boiling point and therefore is less volatile than nitrogen and argon. 1 Air is filtered to remove particulates.
 Source: byjus.com
Source: byjus.com
The process takes advantage of the fact that when a compressed gas is allowed to expand it cools. Energy of first ionisation. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. They give the temperature at which the vapor pressure of the liquid is equal to atmospheric pressure at sea. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds.
 Source: chemsrc.com
Source: chemsrc.com
It is resistant to dilute sulfuric and hydrochloric acids moist chlorine gas most organic acids and chloride solutions. They give the temperature at which the vapor pressure of the liquid is equal to atmospheric pressure at sea. Its minerals are widely distributed with pyrolusite manganese dioxide and rhodochrosite manganese carbonate being the most common. This must be because the boiling point of water falls with decreasing atmospheric pressure P atm. Manganese Dioxide 3 CAS 1313-13-9 5 mgm3 Ceiling as Mn 02 mgm TWA as Mn 30-45 Potassium Hydroxide CAS 1310-58-3 None established 2 mgm3 Ceiling 4-8 Zinc 3 CAS 744066- -6 15 mgm TWA PNOR total dust 5 mgm3 TWA PNOR respirable fraction 10 mgm3 TWA PNOC inhalable particulate 3 mgm3 TWA PNOC respirable particulate 12-25.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Manganese dioxide is used as the cathode material in dry-cell batteries. Titanium is only ductile when it is. 59 C 426 F. A WORLD CLASS REFINERY. Aeration brings water and air in close contact by exposing drops or thin sheets of water to the air or by.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title manganese dioxide boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.