Magnesium chloride melting point
Home » datasheet » Magnesium chloride melting pointMagnesium chloride melting point
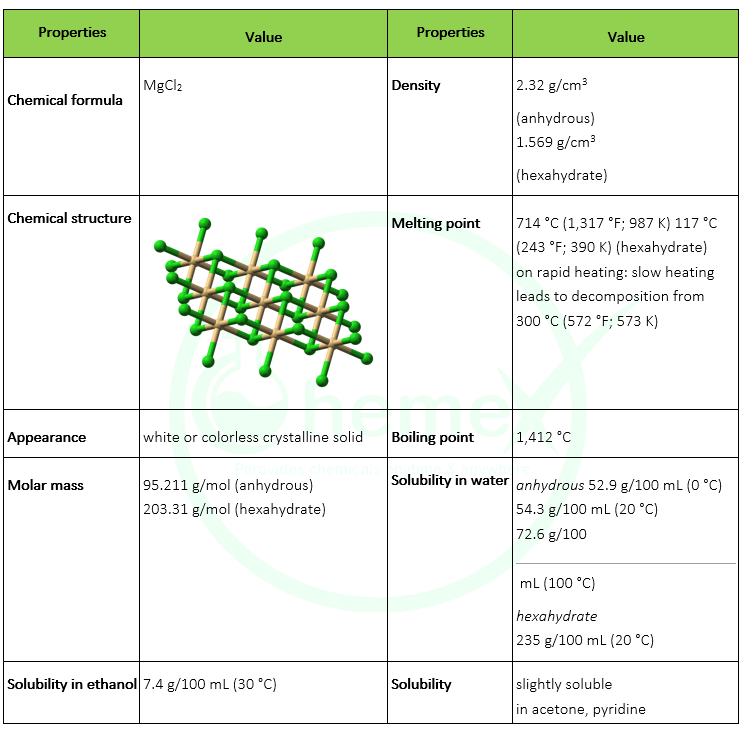
Magnesium Chloride Melting Point. Crisco by partial hydrogenation of their unsaturated components. Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass. Pick up and remove spilled solid before adding waterIt is used to treat sewage industrial waste to purify water as an etching agent for engraving circuit boards and in the manufacture of other chemicals. Ropp in Encyclopedia of the Alkaline Earth Compounds 2013 Calcium Chloride.
 Magnesium Chloride Hexahydrate Shanghai Chemex From shanghaichemex.com
Magnesium Chloride Hexahydrate Shanghai Chemex From shanghaichemex.com
Magnesium nitrate Structure MgNO32. When wet it is corrosive to aluminum and most metals. 923 K 650 C. Melting point of Magnesium nitrate. 1994 F 1090 C. Below the melting point the solid is the more stable state of the two.
All ionic compounds have high melting points for this reason.
Used as a desensitizer for lithographic plates. Used as a desensitizer for lithographic plates. This requires more heat energy to overcome. Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass. It is slightly soluble in waterIt is noncombustible. Calcium chloride CaCl 2 is a typical ionic halide and is a solid at room temperatureIts molecular weight is 11098 gmol and its melting point is 772 CVery few natural minerals occur.
 Source: webelements.com
Source: webelements.com
1994 F 1090 C. Melting point of Magnesium nitrate. 1474 F NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP. These salts are typical ionic halides being highly soluble in waterThe hydrated magnesium chloride can be extracted from brine or sea waterIn North America magnesium chloride is produced primarily from Great. Some of the remaining.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The melting point also defines a condition in which the solid and liquid can exist in equilibrium. National Toxicology Program Chemical Repository Database. Boiling point of Magnesium nitrate. These salts are typical ionic halides being highly soluble in waterThe hydrated magnesium chloride can be extracted from brine or sea waterIn North America magnesium chloride is produced primarily from Great. Magnesium nitrate Structure MgNO32.

This technique utilized in the Magnetherm process has the advantage that the liquid slag can be heated. The melting point also defines a condition in which the solid and liquid can exist in equilibrium. It is slightly soluble in waterIt is noncombustible. But not produced in metallic form until 1831 when Antoine Bussy made magnesium during an experiment with dehydrated magnesium chloride. Used in the manufacturing of petrochemicals.
 Source: kissner.com
Source: kissner.com
1202 F 650 C Boiling Point. Used as a desensitizer for lithographic plates. Below the melting point the solid is the more stable state of the two. 3600 F and is therefore present as a solid but by adding alumina aluminum oxide Al 2 O 3 to the charge the melting point can be reduced to 15501600 C 28252900 F. This technique utilized in the Magnetherm process has the advantage that the liquid slag can be heated.
 Source: slideplayer.com
Source: slideplayer.com
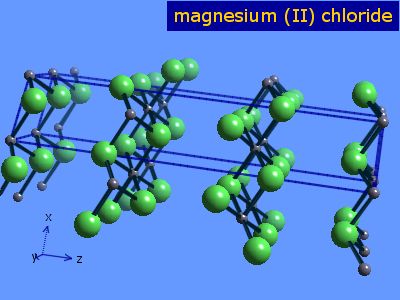
But not produced in metallic form until 1831 when Antoine Bussy made magnesium during an experiment with dehydrated magnesium chloride. Magnesium chloride is recoverable from naturally occurring brines such as the Great Salt Lake. From magnesium chloride electrolysis produces magnesium. The occurrence of a dihydrate mineral Sinjarite and hexahydrate Antarcticite is very rare and is connected. Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass.
 Source: scbt.com
Source: scbt.com
National Toxicology Program Chemical Repository Database. Ferric chloride is an orange to brown-black solid. Used in the manufacturing of petrochemicals. Commercial production of electrolytic magnesium began in Germany in 1886. This requires more heat energy to overcome.
923 K 650 C. The occurrence of a dihydrate mineral Sinjarite and hexahydrate Antarcticite is very rare and is connected. The melting point also defines a condition in which the solid and liquid can exist in equilibrium. The thermal and electrical conductivity of magnesium and its melting point are very similar to those of aluminum. MgNO 3 2 Uses Magnesium nitrate Magnesium nitrate is used as a dehydrating agent to prepare concentrated nitric acid.
 Source: en.wikipedia.org
Source: en.wikipedia.org
All ionic compounds have high melting points for this reason. 1202 F 650 C Boiling Point. Commercial production of electrolytic magnesium began in Germany in 1886. However after alloying Mg with 1 Al and 01 Ca its thickness could be reduced by 54 using the same process bottom. Magnesium chloride is recoverable from naturally occurring brines such as the Great Salt Lake.
 Source: shanghaichemex.com
Source: shanghaichemex.com
1474 F NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP. Pick up and remove spilled solid before adding waterIt is used to treat sewage industrial waste to purify water as an etching agent for engraving circuit boards and in the manufacture of other chemicals. Natural mixed triglycerides have somewhat lower melting points the melting point of lard being near 30 º C whereas olive oil melts near -6 º C. These salts are typical ionic halides being highly soluble in waterThe hydrated magnesium chloride can be extracted from brine or sea waterIn North America magnesium chloride is produced primarily from Great. Research Triangle Park North Carolina.
 Source: pediaa.com
Source: pediaa.com
The occurrence of a dihydrate mineral Sinjarite and hexahydrate Antarcticite is very rare and is connected. Calcium chloride CaCl 2 is a typical ionic halide and is a solid at room temperatureIts molecular weight is 11098 gmol and its melting point is 772 CVery few natural minerals occur. The country remained the only producer until 1916 when military demand for magnesium for flares and. 1202 F 650 C Boiling Point. Below the melting point the solid is the more stable state of the two.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title magnesium chloride melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.