Magnesium boiling point
Home » datasheet » Magnesium boiling pointMagnesium boiling point
Magnesium Boiling Point. Melting point - the temperature at which a solid turns into a liquid. Magnesium carbonate is a magnesium salt with formula CMgO3. Place the magnesium chloride flakes in the glass bowl or measuring cup and the pour the boiling water over it. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure.
 Magnesium From onyxmet.com
Magnesium From onyxmet.com
Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass. 7837 C 1731 F Boiling point of methanol. Click on any elements name for further chemical properties environmental data or health effects. Note that these points are associated with the standard atmospheric pressure. 12 Number of Neutrons. Place the magnesium chloride flakes in the glass bowl or measuring cup and the pour the boiling water over it.
It is the temperature at which the solid phase changes to the liquid phaseThis is the point at which both liquid and solid phases exist at equilibriumVisit BYJUS to learn more about the Principle Detailed Explanation Videos and FAQs of melting point and Boiling point.
The chemical elements of the periodic chart sorted by. 24305 amu Melting Point. Sodium magnesium and aluminium are all metals. Let cool completely and store in the spray bottle. The metal can be produced artificially but is highly reactive. Atomic number - Name alphabetically-269.
 Source: researchgate.net
Source: researchgate.net
That is because of the significant magnesium content in salt water which averages about 1290 parts per million ppm. Pure polycrystalline magnesium is brittle and easily fractures along shear bands. Atomic number - Name alphabetically-269. Theyre exceptionally high in magnesium. 7837 C 1731 F Boiling point of methanol.
 Source: pinterest.com
Source: pinterest.com
Occurrence properties and uses. But when water bodies are accounted for magnesium becomes the most abundant element on the surface of the earth. In general boiling is a phase change of a substance from the liquid to the gas phase. It is a magnesium salt a carbonate salt and a one-carbon compound. We have been using Magnesium since a hundred years in.

-269 C -452 F. 1 ounce thats around 142 seeds deliver 168 mg. Note that these points are associated with the standard atmospheric pressure. Magnesium - Melting Points of Binary Eutectic Alloys - Mg - Magnesium - binary eutectic alloys and melting points. It has a role as an antacid and a fertilizer.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
174 at 20 C 68 F oxidation state 2. 650 C 1202 F boiling point. Elemental magnesium is a gray-white lightweight metal and occurs naturally only in combination with other elements. Lowest melting and boiling points elements in a period. Magnesium is the eighth most abundant element in the Earths crust but does not occur uncombined in nature.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Elemental magnesium is a gray-white lightweight metal and occurs naturally only in combination with other elements. Magnesium is classified as an alkaline earth metal and has 2 hydration shells. I keep in my bathroom to use daily. Melting point of Magnesium is 649C. 56 C 1328 F Boiling point of alcohol.
 Source: researchgate.net
Source: researchgate.net
Magnesium is the eighth most abundant element in the Earths crust. Melting point - the temperature at which a solid turns into a liquid. 650 C 1202 F boiling point. Magnesium is used in super-strong lightweight. Pumpkin and squash seeds are an extremely nutritious snack.
 Source: material-properties.org
Source: material-properties.org
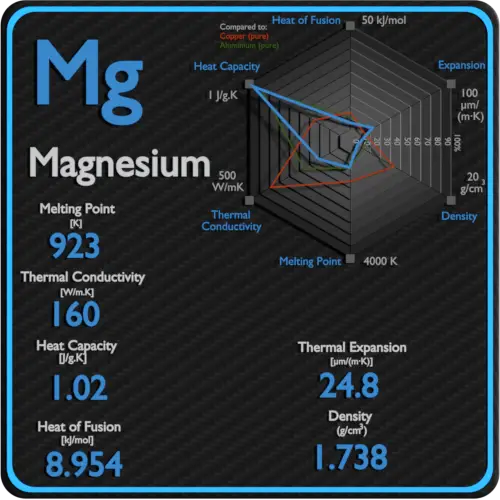
1738 gcm 3 Color. Melting Point and Boiling point- Melting point is a characteristic property of solid crystalline substances. Stir well until completely dissolved. As can be seen the boiling point of a liquid varies depending upon the surrounding environmental pressure. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
 Source: youtube.com
Source: youtube.com
Magnesium is the most chemically active element. Melting point - the temperature at which a solid turns into a liquid. Boiling point of Magnesium is 1090C. 24305 amu Melting Point. In boiling water the place of hydrogen is taken by Magnesium and a number of metals can be produced using thermal reduction of its salts and oxidized forms with magnesium.
 Source: chemguide.co.uk
Source: chemguide.co.uk
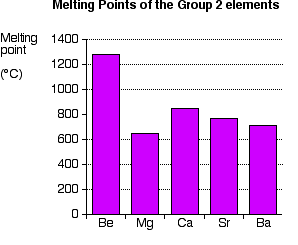
But when water bodies are accounted for magnesium becomes the most abundant element on the surface of the earth. Click on any elements name for further chemical properties environmental data or health effects. I keep in my bathroom to use daily. Magnesium has the lowest melting 923 K 1202 F and the lowest boiling point 1363 K 1994 F of all the alkaline earth metals. The number of delocalised electrons increases.
 Source: onyxmet.com
Source: onyxmet.com
If you come across an explanation for the very small increase in melting point from magnesium to aluminium in terms of the strength of the metallic bond you should be very wary of it unless it also explains why despite that the boiling point of aluminium is much higher than that of magnesium. Permanent hardness is generally associated with calcium sulfate andor magnesium sulfates in the water which will not precipitate when the water is boiled. Can be stored at room temperature for at least six months. But when water bodies are accounted for magnesium becomes the most abundant element on the surface of the earth. Sodium magnesium and aluminium.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title magnesium boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.