Lead acetate boiling point
Home » datasheet » Lead acetate boiling pointLead acetate boiling point
Lead Acetate Boiling Point. Thanksyou made it extremely understandable. The treatment and conditioning of boiler feed water must satisfy three main objectives. Its boiling point of 1749 C 3180 F is the lowest among the carbon group elements. A complementary stereoselective reduction in the anti mode.
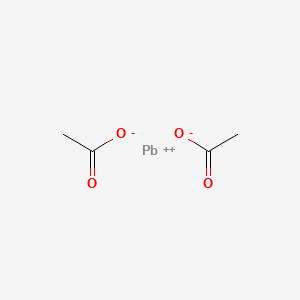
Lead Ii Acetate C4h6o4pb Chemspider From chemspider.com
8829 C 1621 F specific gravity. External treatment is the reduction or removal of impurities from water outside the boiler. The addition of hydrogen is stereoselectively syn eg. The main target users are workers and those responsible for occupational safety and health. Lead MCL 0015 mgL EPA US 2006. The basic principle described as the input of heat energy raises vapor pressure.
7837 C 1731 F Boiling point of methanol.
Thus the distillation process depends on the vapor pressure characteristics of liquid mixtures. Copies may be obtained from the American Society for Testing Materials 100 Barr Harbor Dr West Conshohocken Philadelphia PA 19428-2959 or may be examined at the National Archives and Records Administration NARA. A thin stick of such material. Production of high quality steam. Thus the distillation process depends on the vapor pressure characteristics of liquid mixtures. In 1807 Sir Humphry Davy became the first to prepare sodium in its elemental form.
Source: chemspider.com
Apart from these primary branches there exist several specialized fields of chemistry that deal with cross-disciplinary matters. In general external treatment is used when the amount of one or more of the feed water impurities is too high to be tolerated by the. 56 C 1328 F Boiling point of alcohol. In 1807 Sir Humphry Davy became the first to prepare sodium in its elemental form. 8829 C 1621 F specific gravity.
 Source: en.wikipedia.org
Source: en.wikipedia.org
May 6 2016 at 1134 am. Ethyl acetate and acetonitrile are better than acetone for this purpose because water can be more easily removed by salting out. Its boiling point of 1749 C 3180 F is the lowest among the carbon group elements. Because sodium is extremely reactive it never occurs in the free state in Earths crust. Nice really appreciate your broadness without sticking to a job.
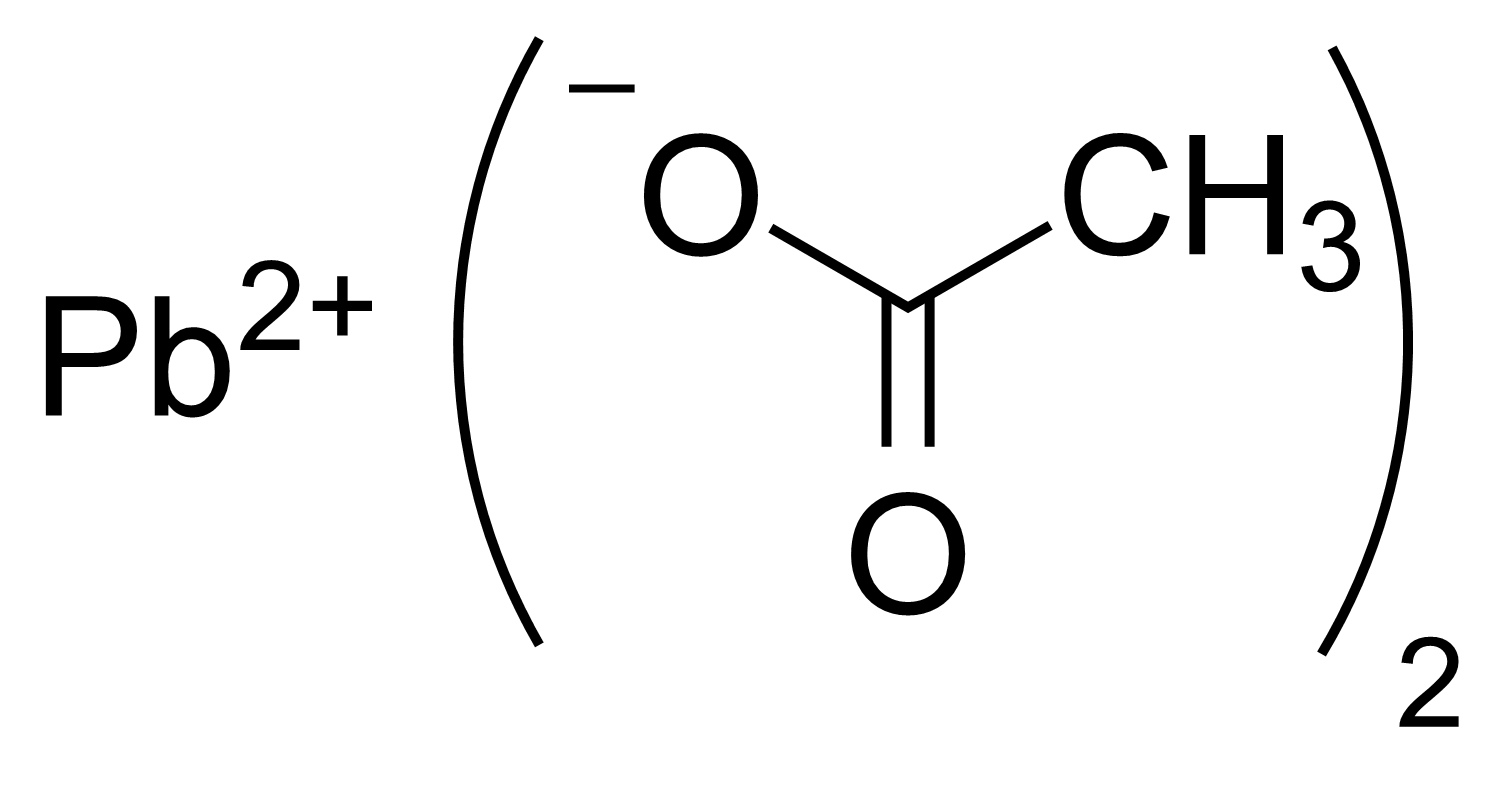 Source: en.wikipedia.org
Source: en.wikipedia.org
Commonly used solvents like ethyl acetate 81 diethyl ether 69 dichloromethane 13 and chloroform 08 dissolved up to 10 in water. Lead definition to go before or with to show the way. -1958 C -3204 F Boiling point of liquid helium. 647 C 1485 F Boiling point of acetone. Visit ChemicalBook To find more Ethyl acetate141-78-6 information like chemical propertiesStructuremelting pointboiling pointdensitymolecular formulamolecular weight physical propertiestoxicity informationcustoms codes.
 Source: wikiwand.com
Source: wikiwand.com
Ethyl acetate 3 diethyl ether 14 dichloromethane 025 and chloroform 0056. Ethyl acetate and acetonitrile are better than acetone for this purpose because water can be more easily removed by salting out. Some such examples include medicinal chemistry neurochemistry materials chemistry nuclear chemistry environmental chemistry polymer chemistry and thermochemistry. The authors reported that the invented technique can successfully extract lavender oil from the dried lavender inflorescences. Briefly the ohmic-evaporated H 2 O molecules from the boiling flask reached the lavender flask extracted volatile components and transferred them to the cooling section.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Determination of boiling-point range. The basic principle described as the input of heat energy raises vapor pressure. Boiling point of water. Apart from these primary branches there exist several specialized fields of chemistry that deal with cross-disciplinary matters. The addition of hydrogen is stereoselectively syn eg.
Source: researchgate.net
A less efficient catalyst Lindlars catalyst prepared by deactivating or poisoning a conventional palladium catalyst by treating it with lead acetate and quinoline permits alkynes to be converted to alkenes without further reduction to an alkane. Pumped the target full of lead. A lead weight suspended by a line used to make. Any of various often graphitic compositions used as the writing substance in pencils. 647 C 1485 F Boiling point of acetone.
In 1807 Sir Humphry Davy became the first to prepare sodium in its elemental form. For more information about the natural variations of the atomic weight of lead please read IUPAC Technical Report Variation of lead isotopic composition and atomic weight in terrestrial materials IUPAC Technical Report. Different forms of lead and particle size have different rates of absorption in the rat. To lead a group on a cross-country hike. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way.
 Source: commons.wikimedia.org
Source: commons.wikimedia.org
Nonpolar cosolvents are needed in the case of acetone which leads to dilution of the extract and greater coextraction of lipids. The ICSC project is a common undertaking between the World Health Organization WHO and. The addition of hydrogen is stereoselectively syn eg. 7837 C 1731 F Boiling point of methanol. Ethyl acetate also extracts lipids readily which makes acetonitrile the most advantageous solvent in multiclass multiresidue methods.
 Source: commons.wikimedia.org
Source: commons.wikimedia.org
Thanks a lot Reply. Ethyl acetate also extracts lipids readily which makes acetonitrile the most advantageous solvent in multiclass multiresidue methods. Ethyl acetate 3 diethyl ether 14 dichloromethane 025 and chloroform 0056. Use ASTM method D86-82 Standard Method for Distillation of Petroleum Products which is incorporated by reference. Copies may be obtained from the American Society for Testing Materials 100 Barr Harbor Dr West Conshohocken Philadelphia PA 19428-2959 or may be examined at the National Archives and Records Administration NARA.
Source: merckmillipore.com
Thus the distillation process depends on the vapor pressure characteristics of liquid mixtures. Some such examples include medicinal chemistry neurochemistry materials chemistry nuclear chemistry environmental chemistry polymer chemistry and thermochemistry. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. Because sodium is extremely reactive it never occurs in the free state in Earths crust. Nonpolar cosolvents are needed in the case of acetone which leads to dilution of the extract and greater coextraction of lipids.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title lead acetate boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.