Isopentyl acetate melting point
Home » datasheet » Isopentyl acetate melting pointIsopentyl acetate melting point
Isopentyl Acetate Melting Point. Crisco by partial hydrogenation of their unsaturated components. CH 3 CH 2 CH 2 COOCH 2 CH 3. Only 1-15 of the administered doses of 3-methyl-1-butanol were excreted in the expired air plus urine as the pentanolThe blood concentration of 3-methyl-1-butanol decreased from 37 mg100 ml at 1 hr ie 15 min after the last pentanol injection to. It derives from an isoamylol.
 Isopentyl Acetate Cas 123 92 2 Scbt Santa Cruz Biotechnology From scbt.com
Isopentyl Acetate Cas 123 92 2 Scbt Santa Cruz Biotechnology From scbt.com
67 cups 161 milliliter of isopropyl alcohol would use 0. BUT do bear in mind it is only PART of the entire formulation. One of our Science teachers has asked for these specific agar plates that are used for culturing microorganisms associated with brewing and. 041 your beer is approximately 8. 25 1 1000 400. The melting point is specific for a given substance.
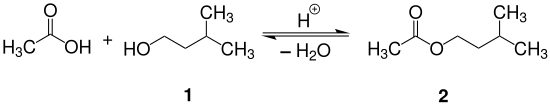
The current experiment uses the carboxylic acid derivative acetic anhydride for ester formation.
Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more. It has a role as a metabolite and a Saccharomyces cerevisiae metabolite. These courses were created by OCTs are tailored specifically to the Ontario curriculum and in light of COVID-19 are 100 free for Ottawa Catholic School Board. Ethyl acetate is the principal one occurring at 1030 mgl with values up to 69 mgl while isopentyl acetate has been found at 178 mgl. Please use this book to increase your knowledge for the laboratory pratictioner. Synthesis of benzocaine mechanism.

NaCl melting point 1074 K C K S 27315 1074 S 27315 80085oC 801oC 9 9 o F x o C 32 x 80085 32 147353o F 1474 o F 5 5 NaCl boiling point 1686 K o C K S 27315 1686 S 27315 141285oC 1413oC 9 9 o F x o C 32 x 141285 32 257513o F 2575o F 5 5 9 9 o F x o C 32 x _ 389 32 _ 380 o F 5 5 o 195 196 V 1125 g x 197 1528 1. CH 3 CH 2 CH 2 COOCH 3. Acetone - Thermophysical Properties - Chemical physical and thermal properties of acetone also called 2-propanone dimethyl ketone and pyroacetic acid. CH 3 COOCH 2 CH 3. Melting Point C Physical Form.
 Source: scbt.com
Source: scbt.com
25 1 1000 400. HOC 6 H 4 COOC 6 H 5. CH 3 COOC 2 H 4 CHCH 3 2-78. NaCl melting point 1074 K C K S 27315 1074 S 27315 80085oC 801oC 9 9 o F x o C 32 x 80085 32 147353o F 1474 o F 5 5 NaCl boiling point 1686 K o C K S 27315 1686 S 27315 141285oC 1413oC 9 9 o F x o C 32 x 141285 32 257513o F 2575o F 5 5 9 9 o F x o C 32 x _ 389 32 _ 380 o F 5 5 o 195 196 V 1125 g x 197 1528 1. It derives from an isoamylol.
 Source: en.wikipedia.org
Source: en.wikipedia.org
CH3C6H3ClNH22 0005 Sk 2-Ethoxyethanol C2H5OCH2CH2OH 10 37 Sk 2-Ethoxyethyl acetate C2H5OCH2CH2OOCCH3 10 54 Sk Ethylene oxide 5 10 Formaldehyde 2 25 2 25 Grain dust See Annexure 7 10 Sen Hydrogen cyanide 10 10 Sk Isocyanate all isomers as NCO 002 007 Sen Lead and compounds- as Pb See the Lead Regulations 2-Methoxyethanol CH3OCH2CH2OH 5 16 Sk 2. Isoamyl acetate also known as isopentyl acetate is an organic compound that is the ester formed from isoamyl alcohol and acetic acid. Only 1-15 of the administered doses of 3-methyl-1-butanol were excreted in the expired air plus urine as the pentanolThe blood concentration of 3-methyl-1-butanol decreased from 37 mg100 ml at 1 hr ie 15 min after the last pentanol injection to. Please use this book to increase your knowledge for the laboratory pratictioner. Hydrogen Balloons Latex 1 Responses.

C 3 H 7 COOC 2 H 5-98. CH 3 COOC 2 H 4 CHCH 3 2-78. We say that such a body melts. Crisco by partial hydrogenation of their unsaturated components. C 3 H 7 COOC 2 H 5-98.
 Source: mccordresearch.com.au
Source: mccordresearch.com.au
HOC 6 H 4 COOC 6 H 5. Iso-amyl acetate is an oily liquid. Sign in to respond to this question. C 3 H 7 COOC 2 H 5-98. 78 C 108 F.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Boiling Point C Feature. Assay minimum 98 percent dry basis. The easiest way to buy some rubbing alcohol is on Amazon. Only 1-15 of the administered doses of 3-methyl-1-butanol were excreted in the expired air plus urine as the pentanolThe blood concentration of 3-methyl-1-butanol decreased from 37 mg100 ml at 1 hr ie 15 min after the last pentanol injection to. Sign in to respond to this question.
 Source: wikidata.org
Source: wikidata.org
Assay minimum 98 percent dry basis. Since fats are valued over oils by some Northern European and North American populations vegetable oils are extensively converted to solid triglycerides eg. Floats and mixes with water. The advantage of using. 195 K Boiling point.
Melting Point C Physical Form. SYNTHESIS OF ISOPENTYL ACETATE BANANA OIL The reaction of a carboxylic acid and an alcohol produces an ester and water. CH 3 COOC 2 H 4 CHCH 3 2-78. 041 your beer is approximately 8. C 3 H 7 COOC 2 H 5-98.
 Source: chemsrc.com
Source: chemsrc.com
Some facts The melting point is the highest temperature at which crystallization may occur. C and compare it to a sample of pure salicylic acid. HOC 6 H 4 COOC 6 H 5. Analytical 4 ACS reagent 2 BioReagent 2 Technique. 3-Methyl-1-butanol following serial four 15-minute intervals ip injections in the rat is very rapidly metabolized.
 Source: en.wikipedia.org
Source: en.wikipedia.org
195 K Boiling point. Isoamyl acetate also known as isopentyl acetate is an organic compound that is the ester formed from isoamyl alcohol and acetic acid. Sodium salt of dehydroacetic acid. The amount of phenethyl acetate varies between 0. Acetone - Thermophysical Properties - Chemical physical and thermal properties of acetone also called 2-propanone dimethyl ketone and pyroacetic acid.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title isopentyl acetate melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.