Isobutane boiling point
Home » datasheet » Isobutane boiling pointIsobutane boiling point
Isobutane Boiling Point. C 2 H 6-88. Feb 12 19 at 2012. Isobutane is a colorless gas with a faint petroleum-like odor. It is the simplest alkane with a tertiary carbon atom.
 Why Butane Exist As A Liquid In A Lighter Chemistry Stack Exchange From chemistry.stackexchange.com
Why Butane Exist As A Liquid In A Lighter Chemistry Stack Exchange From chemistry.stackexchange.com
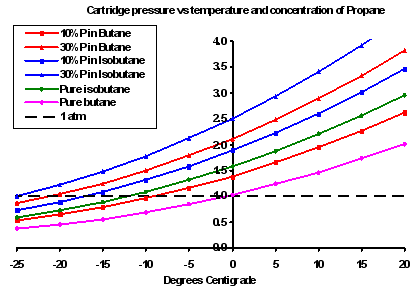
This helped me a lot to understandi have visited many sites before but it is the most useful 1thanks. Low boiling point fluids such as CFC and HFCF early days. Isobutane and butane have different boiling points the temperature at which it goes from liquid to gas vapour. Isobutane belongs to the hydrocarbon refrigerant classification and it along with propane are the most popular hydrocarbon refrigerants used today. So you could find. No Boiling No Vapourisation No Gas.
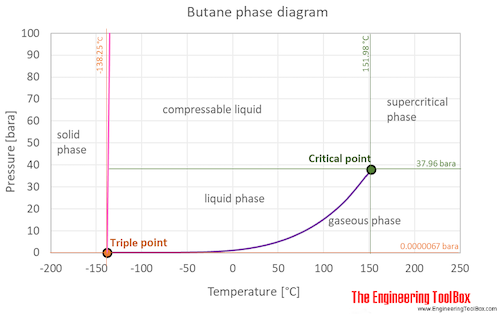
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and.
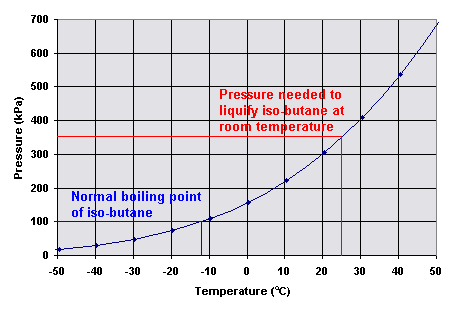
March 12 2012 at 245 pm. Glad you found it useful sumaiya. Isobutane boils at -1175C whereas butane boils at -04C. 2-methylpropane isobutane CH 3 CHCH 3CH 3-117. The boiling points of the alkanes gradually increase with the. C 2 H 6-88.
 Source: bushwalkingnsw.org.au
Source: bushwalkingnsw.org.au
The boiling points of the alkanes gradually increase with the. 2614 K Solubility in water. C 4 H 8. Isobutane is a colorless gas with a faint petroleum-like odor. Because the branched-chain isomer has a smaller surface area you could predict a lower boiling point when compared with straight-chain butane.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It is shipped as a liquefied gas under its vapor pressure. R-600a is used for blending in a variety of other refrigerants mixes found in HCFC HFC and Hydrocarbon. It is the simplest alkane with a tertiary carbon atom. In mice exposed to a liquid gas mixture containing propane butane isobutane at 17 31 52 respectively death occurred within 15 seconds of exposure. Concn of the cmpd in lung tissue were max within 1 hr of death decreased thereafter depending on the degree of putrefaction.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
I just search it on wikipedia. Acetic acid anhydride CH 3 COO 2 O. 2614 K Solubility in water. The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below. Because isomers are different compounds they can have different physical and chemical properties.
 Source: researchgate.net
Source: researchgate.net
The boiling point of a substance is the temperature at which it can change state from a liquid to a gas throughout the bulk of the liquid. Pentane 2-methylbutane 22-dimethylpropane Molecular. It is an isomer of butane. It is the simplest alkane with a tertiary carbon atom. C 3 H 4-232.
 Source: digipac.ca
Source: digipac.ca
C 6 H 14. 2614 K Solubility in water. Acetone CH 3 COCH 3. March 12 2012 at 244 pm. R-600a is used for blending in a variety of other refrigerants mixes found in HCFC HFC and Hydrocarbon.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
The four-carbon alkane is butane with the formula C 4 H 10. No residues or only traces were detected by the 15th day postmortem. Isobutane is used as a precursor molecule in the petrochemical. Max concn were observed in the adipose. Indeed isobutane has a boiling point of -13C while butane has a boling point of -1C.
 Source: pediaa.com
Source: pediaa.com
C 6 H 14. Contact with the liquid can cause frostbite. 1 begingroup Yet the pentanes all boil higher than the butanes according to MaxWs list. Isobutane is used as a precursor molecule in the petrochemical. Isobutane 75-28-5 X X X - CERCLA SARA SARA 311312 Hazard Categories Immediate.
 Source: physics.stackexchange.com
Source: physics.stackexchange.com
Glad you found it useful sumaiya. The compounds n-butane and isobutane are constitutional isomers and are the only ones possible for the formula C 4 H 10. Pentane n -pentane chemical formula is C 5 H 12 but is only a gas over 361C. C 6 H 12. Because isomers are different compounds they can have different physical and chemical properties.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Product Boiling Point at Atmospheric Pressure o C Acetaldehyde CH 3 CHO. The ejector cooling system has been. Isobutane boils at -1175C whereas butane boils at -04C. The working fluid circulates within a closed cycle generating an amount of net power equal to the difference between the power produced by the turbine and the power needed to run the well pump the working-fluid condensate pump and the. It can asphyxiate by the displacement of air.
 Source: thermopedia.com
Source: thermopedia.com
A Compressed gases B5 Flammable aerosol. March 12 2012 at 244 pm. The heat extracted from the geofluid is used to preheat and then evaporate a low-boiling-point working fluid typically a hydrocarbon or other organic fluid. For example n-butane has a higher boiling point 05 C 311 F than isobutane 117 C 109 F. Feb 12 19 at 2012.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title isobutane boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.