Iron iii chloride melting point
Home » datasheet » Iron iii chloride melting pointIron iii chloride melting point
Iron Iii Chloride Melting Point. FeCl 3 Fe 2 O 3 3FeOCl. Special Warnings from DuPont. Iron Melting Point and Boiling Point. The compound is white but typical samples are often off-white.
Iron Iii Chloride Hexahydrate H12cl3feo6 Chemspider From chemspider.com
Ferrous chloride solution is the greenish-white crystalline solid dissolved in water. By reflected light the crystals appear dark green but by transmitted light they appear purple-red. Pick up and remove spilled solid before adding waterIt is used to treat sewage industrial waste to purify water as an etching agent for engraving circuit boards and in the manufacture of other chemicals. FeCl 3 Fe 2 O 3 3FeOCl. Special Warnings from DuPont. Its melting point is 3076 in its anhydrous form.
However due to the presence of contaminants ironIII chloride it acquires a yellowish colour.
FeCl 2 crystallizes from water as the greenish tetrahydrate which is the form that is most commonly encountered in commerce and the laboratory. Liquid 480 480 indicates greater than. Melting point of Iron is 1538C. It is highly soluble in methanol and diethyl ether. Iron is a chemical element in the periodic table that has the symbol Fe and atomic number 26. It is slightly soluble in waterIt is noncombustible.
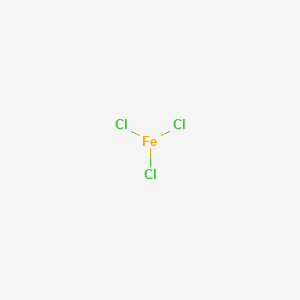
FeCl 2 crystallizes from water as the greenish tetrahydrate which is the form that is most commonly encountered in commerce and the laboratory. Serged and bound seams are degraded by some hazardous liquid chemicals such as strong acids and should not be worn when these chemicals are present. C freezing D melting ii Change of state 4 is called 1 A boiling B condensing C freezing D melting P43530A0536 5 Turn over c The term sublimation is also used for a change of state. When wet it is corrosive to aluminum and most metals. Ferrous chloride is a.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Alloy add-ons also suppress the melting range lower. Serged and bound seams are degraded by some hazardous liquid chemicals such as strong acids and should not be worn when these chemicals are present. When wet it is corrosive to aluminum and most metals. Ferrous chloride is a. The melting point also defines a condition in which the solid and liquid can exist in equilibrium.
 Source: fishersci.co.uk
Source: fishersci.co.uk
Alloy add-ons also suppress the melting range lower. However due to the presence of contaminants ironIII chloride it acquires a yellowish colour. Ferrous chloride is a. C freezing D melting ii Change of state 4 is called 1 A boiling B condensing C freezing D melting P43530A0536 5 Turn over c The term sublimation is also used for a change of state. It is used in dyeing in medicine and sewage treatment.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
When wet it is corrosive to aluminum and most metals. Iron is a chemical element in the periodic table that has the symbol Fe and atomic number 26. Ferric chloride is an orange to brown-black solid. Pick up and remove spilled solid before adding waterIt is used to treat sewage industrial waste to purify water as an etching agent for engraving circuit boards and in the manufacture of other chemicals. Adding a heat will convert the solid into a liquid with no temperature change.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Ferrous chloride is a. Iron is a chemical element in the periodic table that has the symbol Fe and atomic number 26. Pick up and remove spilled solid before adding waterIt is used to treat sewage industrial waste to purify water as an etching agent for engraving circuit boards and in the manufacture of other chemicals. Iii In which state are the particles arranged in a regular pattern. Ferric chloride shows following chemical reactions Reaction with iron III oxide.
Source: chemspider.com
Melting point of Iron is 1538C. Alloy add-ons also suppress the melting range lower. It shows 316 boiling point in its anhydrous form. FeCl 2 crystallizes from water as the greenish tetrahydrate which is the form that is most commonly encountered in commerce and the laboratory. Ferric chloride is an orange to brown-black solid.

Melting point of Iron is 1538C. Pure iron Fe has a fixed melting point of 1535 C chromium Cr of 1890 C and nickel Ni of 1453 C compared to 1400-1450 C for stainless steel of type 304. Below the melting point the solid is the more stable state of the two. IronIII chloride 50 ww in water 7705-08-0. At the melting point the two phases of a substance liquid and vapor have identical free energies and therefore are equally likely to exist.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Solid aluminium chloride AlCl 3 is covalently bonded with low melting as well as boiling point. Below the melting point the solid is the more stable state of the two. It is highly soluble in methanol and diethyl ether. FeCl 2 crystallizes from water as the greenish tetrahydrate which is the form that is most commonly encountered in commerce and the laboratory. In general boiling is a phase change of a substance from the liquid to the gas phase.
Source: chemspider.com
The fabric may or may not offer barrier. The fabric may or may not offer barrier. Boiling point of Iron is 2861C. It is corrosive to metals and tissue. Solid aluminium chloride AlCl 3 is covalently bonded with low melting as well as boiling point.
Source:
FeCl 3 Fe 2 O 3 3FeOCl. Below the melting point the solid is the more stable state of the two. Solid aluminium chloride AlCl 3 is covalently bonded with low melting as well as boiling point. At the melting point the two phases of a substance liquid and vapor have identical free energies and therefore are equally likely to exist. It shows 316 boiling point in its anhydrous form.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title iron iii chloride melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.