Hydrogen sulfide boiling point
Home » datasheet » Hydrogen sulfide boiling pointHydrogen sulfide boiling point
Hydrogen Sulfide Boiling Point. It is heavier than air and may accumulate in low-lying areas. Hydrogen is the lightest element. -60 0 C H 2 O boiling point. Specific tests for the presence of hydrogen sulfide in blood and urine generally are not useful to the doctor.
 Q Why Boiling Point Of Water Is Greater Than Hydrogen Sulphide Class 12 P Block Youtube From youtube.com
Q Why Boiling Point Of Water Is Greater Than Hydrogen Sulphide Class 12 P Block Youtube From youtube.com
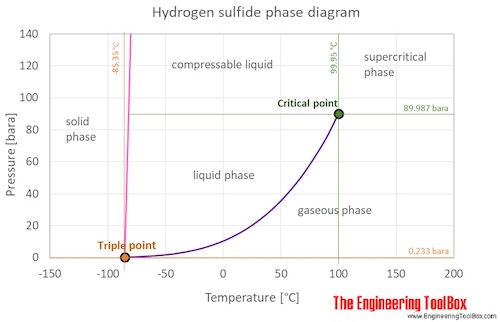
Contact with the unconfined liquid can cause frostbite by evaporative cooling. 301 - 033 P 0045 and the BaxBcl-2 ratio decreased in the model-hydrogen group. It smells like rotten eggs at low concentrations and causes you to quickly lose your sense of smell. It is colorless odorless non-toxic and highly combustible. Boiling point of Hydrogen is -2529C. The curve between the critical point and the triple point shows the hydrogen sulfide boiling point with changes in pressure.
517 - 041 P 0021.
A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. DOT ID Guide. This process is commonly known as anaerobic digestion which is. The boiling points of water and hydrogen sulfide are 100 o C and -60 o C respectively. As can be seen the boiling point of a liquid varies depending upon the surrounding environmental pressure. Synonyms Trade Names Hydrosulfuric acid Sewer gas Sulfuretted hydrogen CAS No.
 Source: researchgate.net
Source: researchgate.net
See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. As can be seen the boiling point of a liquid varies depending upon the surrounding environmental pressure. An explosion occurred upon heating 1-pentol and 1-pentol under hydrogen pressure. 1 ppm 140 mgm 3. First dissociation forms hydronium ion and hydroperoxide ion.
Source: quora.com
Hydrogen Melting Point and Boiling Point. A liquid at high pressure has a higher boiling point than when that liquid is at atmospheric pressure. Density liquid 83 lb gal. At the critical point there is no change of state when pressure is increased or if heat is added. Note that these points are associated with the standard atmospheric pressure.
 Source: youtube.com
Source: youtube.com
It is poisonous corrosive and flammable. Acidic nature of hydrogen peroxide. NIOSH REL C 10 ppm 15 mgm 3 10-minute OSHA PEL C 20 ppm 50 ppm 10-minute maximum peak See Appendix G. Its Ka value is 155 10 -12 mol dm-3 at 298K. -60 0 C H 2 O boiling point.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
For example water boils at 100C 212F at sea level but at 934C 2001. 301 - 033 P 0045 and the BaxBcl-2 ratio decreased in the model-hydrogen group. Hydrogen sulphide is a colourless poisonous gas that can lead to headaches even if it is inhaled in small quantities. Boiling point of Hydrogen is -2529C. It is heavier than air and may accumulate in low-lying areas.
 Source: members.optushome.com.au
Source: members.optushome.com.au
NIST Spectra nist ri. DOT ID Guide. 301 - 033 P 0045 and the BaxBcl-2 ratio decreased in the model-hydrogen group. 1 ppm 140 mgm 3. First dissociation forms hydronium ion and hydroperoxide ion.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
Charlier GO Gas chromatographic separation of hydrogen chloride hydrogen sulfide and water Anal. When a team of researchers tested hydrogen sulfide in asthmatic rats they found that this produced considerable relief. Acidic nature of hydrogen peroxide. Explosive reactions occur upon ignition of mixtures of nitrogen trifluoride with good reducing agents such as ammonia hydrogen hydrogen sulfide or methane. Hydrogen sulfide appears as a colorless gas having a strong odor of rotten eggs.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
An explosion occurred upon heating 1-pentol and 1-pentol under hydrogen pressure. Pure hydrogen peroxide is a weak acid dissociates as follow. A liquid at high pressure has a higher boiling point than when that liquid is at atmospheric pressure. Hydrogen sulfide appears as a colorless gas having a strong odor of rotten eggs. Why H 2 S having less boiling point than H 2 O H 2 S boiling point.
 Source: en.wikipedia.org
Source: en.wikipedia.org
If a severe exposure has occurred blood and urine analyses and other tests may show whether the brain nerves heart or kidneys have been. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. Specific tests for the presence of hydrogen sulfide in blood and urine generally are not useful to the doctor. Acidic nature of hydrogen peroxide. Boiling point - the temperature at which a liquid turns into a gas.
 Source: coolgyan.org
Source: coolgyan.org
This process is commonly known as anaerobic digestion which is. Boiling point H 2 20271 K 252879 C 423182 F Density. It is heavier than air and may accumulate in low-lying areas. Fatigues the sense of smell which. Hydrogen is the most abundant chemical substance in the universe.
 Source: galnet.fandom.com
Source: galnet.fandom.com
Pure hydrogen peroxide is a weak acid dissociates as follow. Chem 393 1967 396-397. DOT ID Guide. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Predicted data is generated using the ACDLabs Percepta Platform - PhysChem.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title hydrogen sulfide boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.