Hydrogen bromide boiling point
Home » datasheet » Hydrogen bromide boiling pointHydrogen bromide boiling point
Hydrogen Bromide Boiling Point. For example phenol molecular weight MW 94 boiling point bp 182 C 3596 F has a boiling point more than 70 degrees higher than that of toluene C 6 H 5 CH 3. Boiling point of water. Hydrogen is the most abundant chemical substance in the universe. The boiling point at atmospheric pressure.
Out Of Hf Hcl Hbr And Hi Which Has The Lowest And Highest Boiling Point And Why Quora From quora.com
Experimental details are in the following. 2 RCH 2 Br NH 3 large excess RCH 2 NH 2 NH 4. Alone of the hydrogen halides hydrogen fluoride exhibits hydrogen bonding between molecules and therefore has the highest melting and boiling points of the HX series. Hydrogen is the chemical element with the symbol H and atomic number 1. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. Hydrogen is the lightest element.
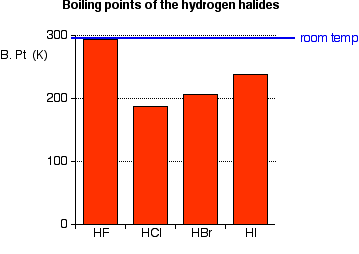
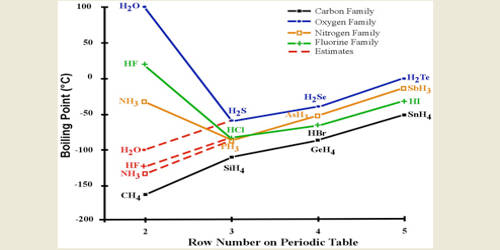
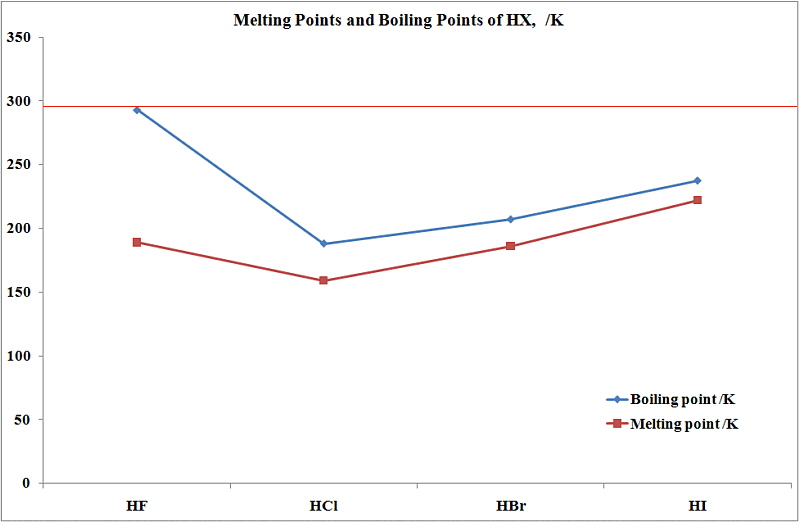
The hydrogen halides are colourless gases at standard conditions for temperature and pressure STP except for hydrogen fluoride which boils at 19 C.
56 C 1328 F Boiling point of alcohol. 7837 C 1731 F Boiling point of methanol. The boiling point of a substance is the temperature at which it changes state from liquid to gas throughout the bulk of the liquid. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Boiling Point ºC-05º. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way.
Source: quora.com
Development of the Table of Initial Isolation and Protective Distances for the 2008 Emergency Response Guidebook ANLDIS-09-2 DF. At standard conditions hydrogen is a gas of diatomic molecules having the formula H 2. Based on a scenario where the chemical is spilled into an excess of water at least 5 fold excess of water half of the maximum theoretical yield of Hydrogen Bromide gas will be created in 002 minutes. The primary aim of the cards is to promote the safe use of chemicals in the workplace. -1958 C -3204 F Boiling point of liquid helium.
Source: quora.com
Experimental details are in the following. At standard conditions hydrogen is a gas of diatomic molecules having the formula H 2. The boiling point at atmospheric pressure. 7837 C 1731 F Boiling point of nitrogen. MW 92 bp 111 C 2318 F.
 Source: chemguide.co.uk
Source: chemguide.co.uk
Hydrogen is the lightest element. At the boiling point molecules anywhere in the liquid may be vaporized. 647 C 1485 F Boiling point of acetone. Boiling point - the temperature at which a liquid turns into a gas. Hydrogen is the chemical element with the symbol H and atomic number 1.
 Source: qsstudy.com
Source: qsstudy.com
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the chemical element with the symbol H and atomic number 1. The main target users are workers and those responsible for occupational safety and health. For example phenol molecular weight MW 94 boiling point bp 182 C 3596 F has a boiling point more than 70 degrees higher than that of toluene C 6 H 5 CH 3. Development of the Table of Initial Isolation and Protective Distances for the 2008 Emergency Response Guidebook ANLDIS-09-2 DF.
Source:
It is colorless odorless non-toxic and highly combustible. We sought to clarify the protective effect of HRS against the oxygen-induced retinopathy OIR in C57BL6 J modelThe OIR in the HRS treated mice and the untreated controls were systematically compared. At the boiling point molecules anywhere in the liquid may be vaporized. The main target users are workers and those responsible for occupational safety and health. For example phenol molecular weight MW 94 boiling point bp 182 C 3596 F has a boiling point more than 70 degrees higher than that of toluene C 6 H 5 CH 3.
Source: en.wikipedia.org
100 C 212 F Boiling point of water in Kelvin. 100 C 212 F Boiling point of water in Kelvin. 3732 K Boiling point of ethanol. Experimental details are in the following. From HCl to HI the.
 Source: favpng.com
Source: favpng.com
EXPL THER Hydrogen-rich saline HRS is a novel protection against various oxidative disorders and almost all types of inflammationMoreover its toxicity and side effects are rarely reported. Boiling point - the temperature at which a liquid turns into a gas. Development of the Table of Initial Isolation and Protective Distances for the 2008 Emergency Response Guidebook ANLDIS-09-2 DF. Water does not normally react with 1º-alkyl halides to give alcohols so the enhanced nucleophilicity of nitrogen relative to oxygen is clearly demonstrated. The main target users are workers and those responsible for occupational safety and health.
 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
From HCl to HI the. 2 RCH 2 Br NH 3 large excess RCH 2 NH 2 NH 4. Water does not normally react with 1º-alkyl halides to give alcohols so the enhanced nucleophilicity of nitrogen relative to oxygen is clearly demonstrated. The ICSC project is a common undertaking between the World Health Organization WHO and. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
7837 C 1731 F Boiling point of nitrogen. The hydrogen halides are colourless gases at standard conditions for temperature and pressure STP except for hydrogen fluoride which boils at 19 C. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. 2 RCH 2 Br NH 3 large excess RCH 2 NH 2 NH 4. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
 Source: sdk.co.jp
Source: sdk.co.jp
The primary aim of the cards is to promote the safe use of chemicals in the workplace. The hydrogen bromide produced in the reaction combines with some of the excess ammonia giving ammonium bromide as a by-product. Hydrogen bonding results in higher melting points and much higher boiling points for phenols than for hydrocarbons with similar molecular weights. Boiling point of water. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title hydrogen bromide boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.