Hydrogen boiling point celsius
Home » datasheet » Hydrogen boiling point celsiusHydrogen boiling point celsius
Hydrogen Boiling Point Celsius. The boiling point is specific for the given substanceFor example the boiling point of. For example the boiling point of pure water at standard atmospheric pressure or sea level is 100C 212F while at 10000 feet 3048m it is 9039 C 1947F. The boiling point temperature will be lower if the atmospheric pressure is decreased. What is the boiling point of BenzaldehydeThe boiling point of Benzaldehyde is 178 degrees Celsius.
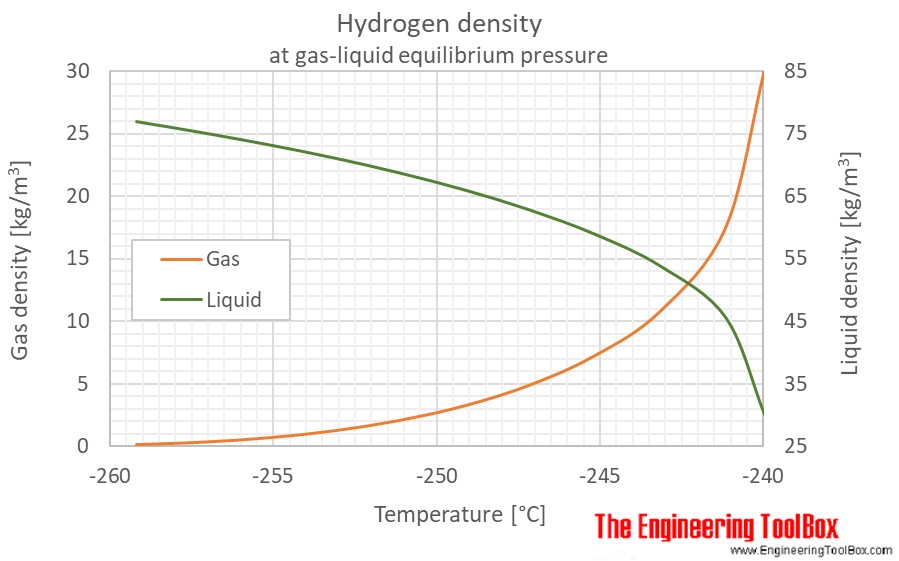 Hydrogen Density And Specific Weight Vs Temperature And Pressure From engineeringtoolbox.com
Hydrogen Density And Specific Weight Vs Temperature And Pressure From engineeringtoolbox.com
100 degrees Celsius is equal to. Heat of Vaporization kJmol 044936. The boiling point at atmospheric pressure. The triple point of a substance is the temperature and pressure at which the three phases gas liquid and solid. For example the boiling point for water at a pressure of 1 atm is 100 degrees Celsius. Heat of Fusion kJmol 005868.
At the critical point there is no change of state when pressure is increased or if heat is added.
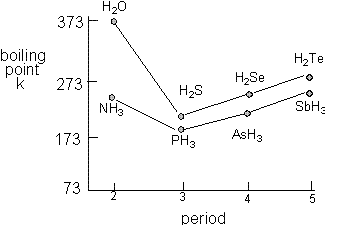
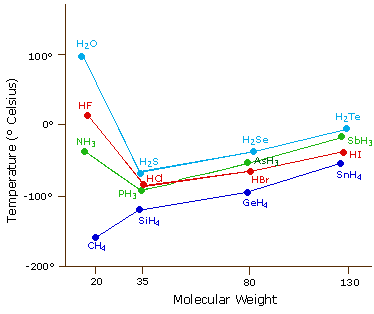
The most powerful intermolecular force influencing neutral uncharged molecules is the hydrogen bondIf we compare the boiling points of methane CH 4 -161ºC ammonia NH 3 -33ºC water H 2 O 100ºC and hydrogen fluoride HF 19ºC we see a greater variation for these similar sized molecules than expected from the data presented above for polar compounds. The Rankine scale is another absolute. Boiling Point Celsius scale-2529. The triple point of a substance is the temperature and pressure at which the three phases gas liquid and solid. Boiling point of water. 3732 K Boiling point of ethanol.
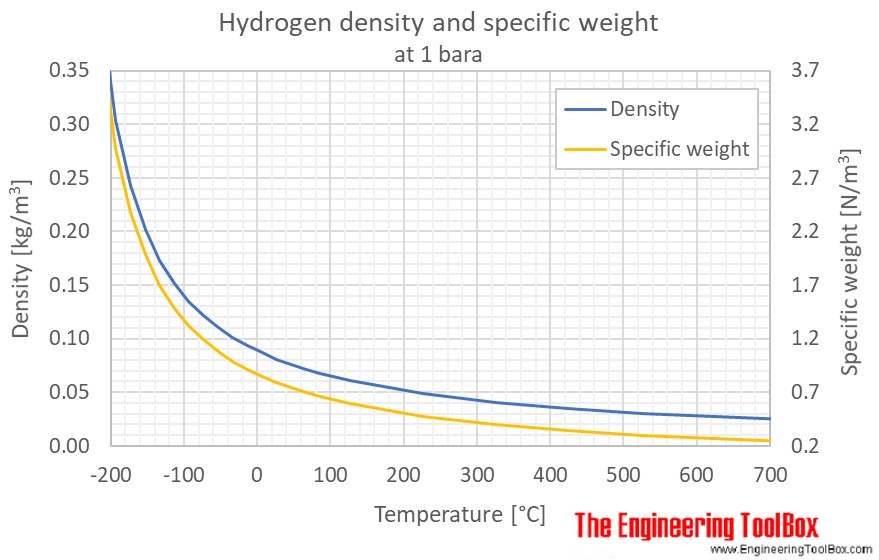 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
H2O2 is miscible with water in all proportions forms a hydrate H2O2H2O. The boiling points of alcohols are much higher than those of alkanes with similar molecular weights. It also shows the saturation pressure with changes in temperature. The unity used for the melting point is Celsius C. For example the boiling point of pure water at standard atmospheric pressure or sea level is 100C 212F while at 10000 feet 3048m it is 9039 C 1947F.
 Source: hydrogen2o2.com
Source: hydrogen2o2.com
In 1742 Swedish astronomer Anders Celsius 17011744 created a temperature scale that was the reverse of the scale now known as Celsius. 56 C 1328 F Boiling point of alcohol. For chemistry students and teachers. The boiling points of alcohols are much higher than those of alkanes with similar molecular weights. The most powerful intermolecular force influencing neutral uncharged molecules is the hydrogen bondIf we compare the boiling points of methane CH 4 -161ºC ammonia NH 3 -33ºC water H 2 O 100ºC and hydrogen fluoride HF 19ºC we see a greater variation for these similar sized molecules than expected from the data presented above for polar compounds.
Thermal Expansion µmmK Thermal Conductivity Wm K 01805. 3732 K Boiling point of ethanol. In this layer the temperature starts decreasing with increasing altitude and reaches up to 100 degree Celsius at the height of 80 km. Hydrogens boiling point is incredibly low at just under 21 degrees Kelvin roughly -421 degrees Fahrenheit liquid hydrogen will turn into a gas. Heat of Vaporization kJmol 044936.
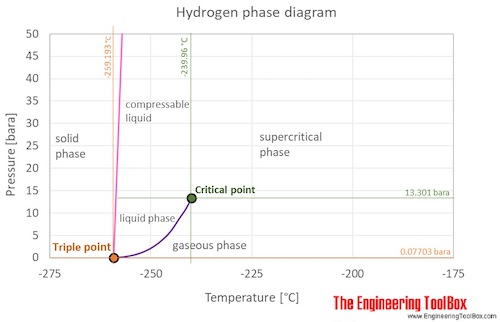 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. These strong intermolecular forces make it difficult to break the molecule apart therefore more energy is needed causing a high melting and boiling point. For example the boiling point of pure water at standard atmospheric pressure or sea level is 100C 212F while at 10000 feet 3048m it is 9039 C 1947F. He found that something flammable. 100 degrees Celsius is equal to.

A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. The tabular chart on the right is arranged by boiling point. 100 degrees Celsius is equal to. The boiling point at atmospheric pressure. A liquids boiling point depends upon the liquid s temperature atmospheric pressure and vapor pressure.
 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
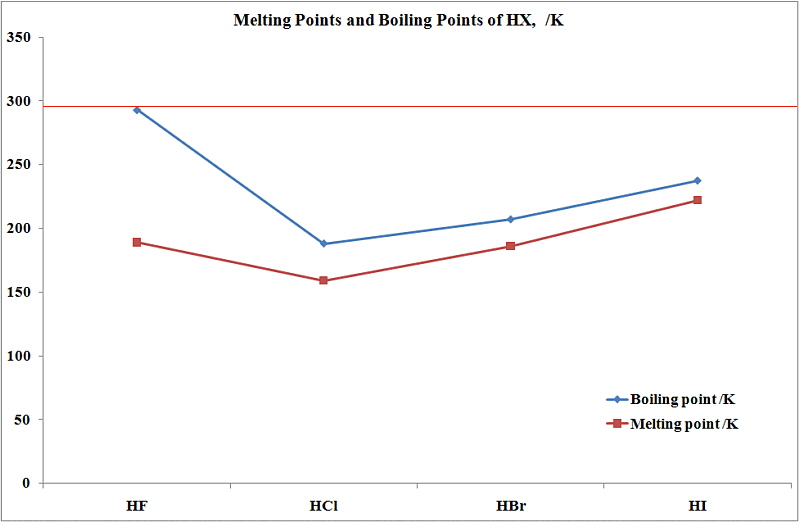
An empirical fit to these data values was made and the formula obtained is shown on the diagram. A liquids boiling point depends upon the liquid s temperature atmospheric pressure and vapor pressure. Boiling Point Celsius scale-2529. Its these hydrogen bonds that give water many of its properties. The boiling point of a liquid varies depending upon the surrounding environmental pressure.
 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. 100 degrees Celsius is equal to. When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils. Melting point 25920 degrees Celsius 25443 degrees Celsius heat of fusion 28 calories per mole 47 calories per mole density of liquid 007099 25278 degrees 01630 24975 degrees boiling point 25277 degrees Celsius 24949 degrees Celsius. Boiling Point Variation Near 100 C Values were taken from the saturated vapor pressure table for water near 100 degrees Celsius.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
In this layer the temperature starts decreasing with increasing altitude and reaches up to 100 degree Celsius at the height of 80 km. Atomic Number of Hydrogen. Melting point 25920 degrees Celsius 25443 degrees Celsius heat of fusion 28 calories per mole 47 calories per mole density of liquid 007099 25278 degrees 01630 24975 degrees boiling point 25277 degrees Celsius 24949 degrees Celsius. What is the boiling point of BenzaldehydeThe boiling point of Benzaldehyde is 178 degrees Celsius. Specific Heat Jg K 14304.
 Source: chemistry.elmhurst.edu
Source: chemistry.elmhurst.edu
The most powerful intermolecular force influencing neutral uncharged molecules is the hydrogen bondIf we compare the boiling points of methane CH 4 -161ºC ammonia NH 3 -33ºC water H 2 O 100ºC and hydrogen fluoride HF 19ºC we see a greater variation for these similar sized molecules than expected from the data presented above for polar compounds. Thermal Expansion µmmK Thermal Conductivity Wm K 01805. 0 degrees Celsius is equal to. The unity used for the melting point is Celsius C. Boiling point of water.

The curve between the critical point and the triple point shows the hydrogen boiling point with changes in pressure. Thermal Expansion µmmK Thermal Conductivity Wm K 01805. The pure state H2O2 is an almost colourless liquid Meting point - 2724K. Liquid water would not feature on the Earth. H2O2 is miscible with water in all proportions forms a hydrate H2O2H2O.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title hydrogen boiling point celsius by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.