Hydrogen boiling point
Home » datasheet » Hydrogen boiling pointHydrogen boiling point
Hydrogen Boiling Point. Even though hydrogen as a pure element bonds to itself to form H 2 its still lighter than a single atom of helium because most hydrogen atoms dont have any neutrons. Atomic number - Name alphabetically-269. Some fuels and their boiling points at atmospheric pressure. The C-O bond dipoles reinforce each other so the molecule has a dipole moment.
 Hydrogen Bonds And Boiling Point From chemistry.elmhurst.edu
Hydrogen Bonds And Boiling Point From chemistry.elmhurst.edu
The presence of hydrogen bonding will lift the melting and boiling points. Most molecular substances are insoluble or only very sparingly soluble in. At standard conditions hydrogen is a gas of diatomic molecules having the formula H 2. The normal boiling point of ethyl alcohol is 785 o C ie a liquid at room temperature. The boiling point of sodium chloride is 2575 F or 1413 C. Dimethyl ether CH_3OCH_3 is a polar molecule.
Due to this relationship between vapor pressure and temperature the boiling point of a liquid decreases as the atmospheric pressure decreases since there is more room above the liquid for molecules to escape into at lower pressure.
It burns when it comes into contact with oxygen. In a liquid the molecules are packed closely together with many random movements possible as molecules slip past each other. 56 C 1328 F Boiling point of alcohol. Hydrogen is the lightest gas. The normal boiling point of ethyl alcohol is 785 o C ie a liquid at room temperature. The boiling point of 785C for ethanol is significantly higher compared with -248C for methoxymethane.
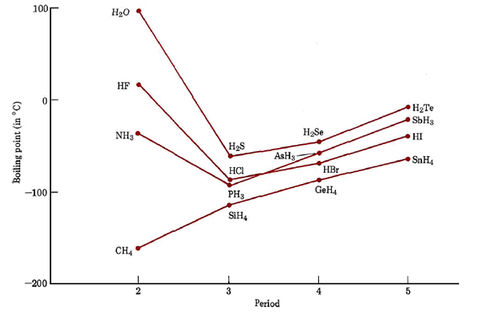 Source: chem.libretexts.org
Source: chem.libretexts.org
Due to this relationship between vapor pressure and temperature the boiling point of a liquid decreases as the atmospheric pressure decreases since there is more room above the liquid for molecules to escape into at lower pressure. In fact two hydrogen atoms 1008 atomic mass units per atom are less than. At standard temperature and pressure hydrogen is a colorless odorless and tasteless gas. The byproduct of a hydrogen and oxygen explosion is water or H 2 O. At standard conditions hydrogen is a gas of diatomic molecules having the formula H 2.
 Source: chemistry.elmhurst.edu
Source: chemistry.elmhurst.edu
But the boiling point of sodium butoxide is higher than that of butanol because the attractive force in sodium butoxide is very strong ionic bond. The normal boiling point of ethyl alcohol is 785 o C ie a liquid at room temperature. 180gmol-1 and yet water has high melting and boiling points. 7837 C 1731 F Boiling point of nitrogen. Hydrogen gas was used in lighter-than-air balloons for transport but is far too dangerous because of the fire risk Hindenburg.

In all three states the same molecules of water H 2 O are present. Explosive reactions occur upon ignition of mixtures of nitrogen trifluoride with good reducing agents such as ammonia hydrogen hydrogen sulfide or methane. Dipole-dipole forces are not as strong as hydrogen bonds so dimethyl ether has a lower boiling point than methanol does. The larger the molecule the more van der Waals attractions are possible - and those will also need more energy to break. The byproduct of a hydrogen and oxygen explosion is water or H 2 O.
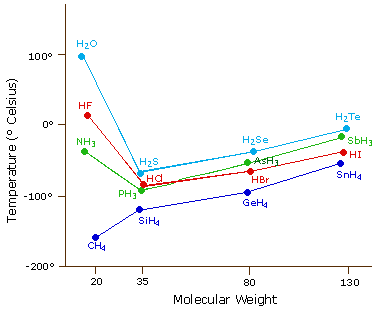 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
This energy can come in the form of heat aka increase in temperature. Most molecular substances are insoluble or only very sparingly soluble in. At 25277 C the pressure exerted by the vapour over liquid para-hydrogen is 1035 atmospheres one atmosphere is the pressure of the. Closely related is the ability of a molecule to form hydrogen bonds in the liquid state which makes it harder for molecules to leave the liquid state and thus increases the normal boiling point of the compound. However there is no danger of boiling the NaCl.
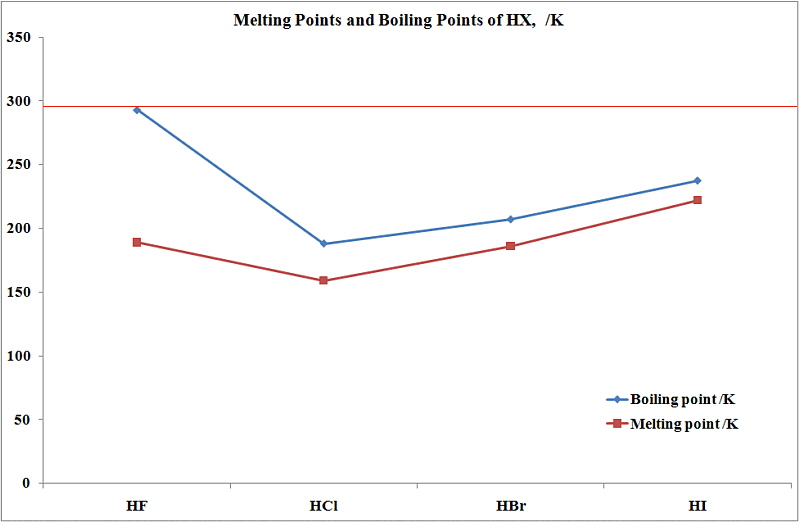 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
Most molecular substances are insoluble or only very sparingly soluble in. 56 C 1328 F Boiling point of alcohol. 2 - Atomic number-253. Hydrogen as water H 2 O is absolutely essential to life and it is present in all organic compounds. It burns in air to form only water as waste product and if hydrogen could be made on sufficient scale from other than fossil fuels then.
 Source: chemistry.elmhurst.edu
Source: chemistry.elmhurst.edu
Due to this relationship between vapor pressure and temperature the boiling point of a liquid decreases as the atmospheric pressure decreases since there is more room above the liquid for molecules to escape into at lower pressure. Hydrogen is the lightest element. Closely related is the ability of a molecule to form hydrogen bonds in the liquid state which makes it harder for molecules to leave the liquid state and thus increases the normal boiling point of the compound. 100 C 212 F Boiling point of water in Kelvin. 180gmol-1 and yet water has high melting and boiling points.
 Source: researchgate.net
Source: researchgate.net
Dipole and hydrogen bonding have to be overcome which requires energy. Boiling Point and Hydrogen Bonding You can check the previous post for more details about the hydrogen bonding. The greatly increased boiling point is due to the fact that butanol contains hydroxyl group which is capable of hydrogen bonding. This example illustrates the significance of bond strength in general and hydrogen. For water the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmHg at 100C.
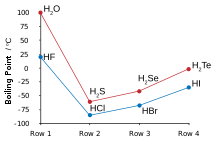 Source: en.wikipedia.org
Source: en.wikipedia.org
At standard temperature and pressure hydrogen is a colorless odorless and tasteless gas. It burns when it comes into contact with oxygen. Even though hydrogen as a pure element bonds to itself to form H 2 its still lighter than a single atom of helium because most hydrogen atoms dont have any neutrons. Here of course the potent intermolecular force of hydrogen bonding operates which was. The curve between the critical point and the triple point shows the hydrogen boiling point with changes in pressure.
 Source: docbrown.info
Source: docbrown.info
Closely related is the ability of a molecule to form hydrogen bonds in the liquid state which makes it harder for molecules to leave the liquid state and thus increases the normal boiling point of the compound. Mixtures of hydrogen carbon monoxide or methane and oxygen difluoride are exploded when a spark is discharged Mellor 2 Supp. As the liquid matter is heated further it eventually boils or vaporizes into a gas at the boiling point. This energy can come in the form of heat aka increase in temperature. Methanol has strong hydrogen bonds.
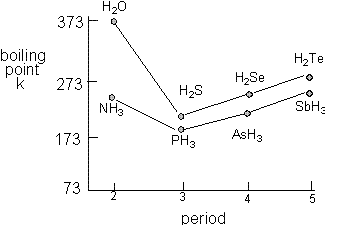
100 C 212 F Boiling point of water in Kelvin. In short it is another type of intermolecular electrostatic interaction that occurs between a hydrogen atom bonded to an electronegative atom such as O N or F is attracted to a lone pair of electrons on an atom in another molecule. 100 C 212 F Boiling point of water in Kelvin. It burns when it comes into contact with oxygen. Since the vapor pressure increases with temperature it follows that for pressure greater than 760 mmHg eg in a pressure cooker the.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title hydrogen boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.