Hydrochloric acid melting point
Home » datasheet » Hydrochloric acid melting pointHydrochloric acid melting point
Hydrochloric Acid Melting Point. It is used in the processing of leather production of gelatin. Hydrochloric acid HCL is a clear colourless and pungent solution created by dissolving hydrogen chloride gas in water. Carolina Biological Supply Company 2700 York Road Burlington NC 27215 1-800-227-1150 Chemical Information. Reacts exothermically with carbonates including limestone and building materials containing limestone and hydrogen carbonates to generate carbon dioxide.
 Hydrochloric Acid Storage Tanks Hcl Specifications From protank.com
Hydrochloric Acid Storage Tanks Hcl Specifications From protank.com
Metallic bismuth is used principally in alloys to many of which it imparts its own special properties of low melting point and expansion on solidification. Hydrochloric acid is an important laboratory reagent and industrial chemical. The melting point is also referred to as liquefaction point solidus or liquidus. He uses dilute hydrochloric acid and solid cobaltII oxide. Amines alkalis copper brass zinc Note. By doing this it will also prevent you or someone else cutting.
Rhenium like platinum in appearance is soluble in dilute nitric acid or hydrogen peroxide but insoluble in hydrochloric acid and hydrofluoric acid.
A substances melting point depends on pressure and is usually specified at standard pressure in reference materials. Step 1 pour about 50 cm3 of dilute hydrochloric acid into a beaker Step 2 warm the acid using a Bunsen burner Step 3 add a small amount of cobaltII oxide and stir the mixture with a glass rod Step 4 add further small amounts of cobaltII oxide until it stops reacting Step 5 filter the final mixture. Reacts exothermically with organic bases amines amides and inorganic bases oxides and hydroxides of metals. Separation of bismuth from its oxide or carbonate ores can be effected by leaching with concentrated hydrochloric acid. 1963C 3565F 2236 K Period 5 Boiling point. Pa ACS Reagent Reag.
 Source: toppr.com
Source: toppr.com
Identification of the substancemixture and of the companyundertaking 11 Product identifiers Product name. Pa ACS Reagent Reag. Keyword 7647-01-0 Showing 1-19 of 19 results for 7647-01-0 within Products. An unknown sample of benzoic acid 2-naphthol and naphthalene Table 1 was massed and the unknown number was recorded. Dilution then precipitates the oxychloride BiOCl.
Melting point The temperature at which the solidliquid phase change occurs. Pa ACS Reagent Reag. Analytical 4 ACS reagent 2 BioReagent 2 Technique. ɪ k is a white or colorless solid with the formula C 6 H 5 CO 2 H. Hydrochloric Acid 6M Page 1 of 4 Hydrochloric Acid 6M Section 1 Product Description Product Name.
 Source: hydro-land.com
Source: hydro-land.com
Recommended use of the chemical and restrictions on use. It is a conjugate acid of a dihydrogenborate. Melting point The temperature at which the solidliquid phase change occurs. Melting point degrees Celsius. Hydrochloric acid is the main component of the gastric juices produced in the stomach and it maintains the stomach pH of 1 to 2 by acidifying the stomach contents.
 Source: researchgate.net
Source: researchgate.net
Rhenium like platinum in appearance is soluble in dilute nitric acid or hydrogen peroxide but insoluble in hydrochloric acid and hydrofluoric acid. 1 Structures Expand this section. Hydrochloric acid is an important laboratory reagent and industrial chemical. Preparation of Hydrochloric acid HCl. Prevents Infections Hydrochloric acid acts as a barrier against foreign microorganisms and helps prevent infection.
 Source: en.wikipedia.org
Source: en.wikipedia.org
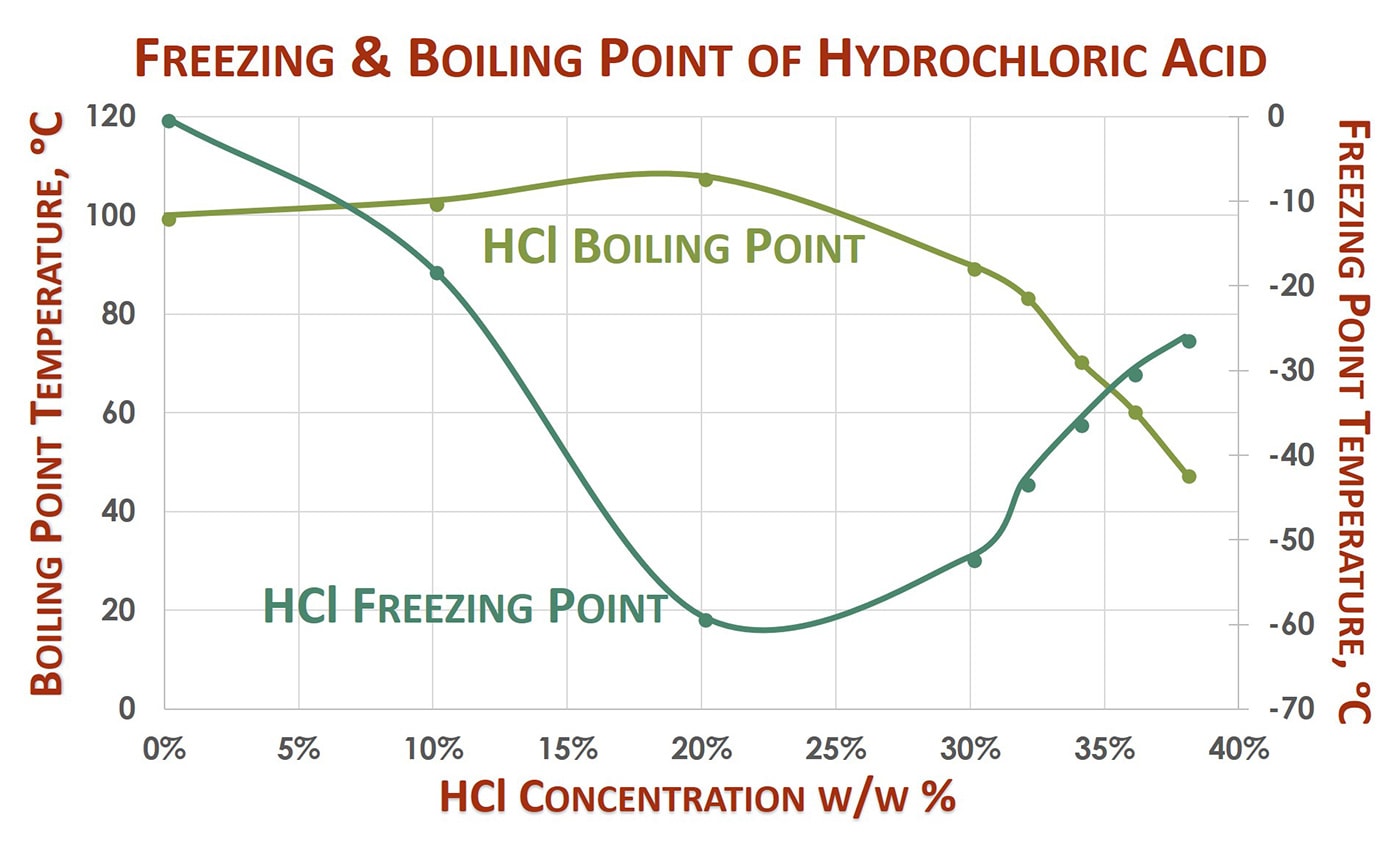
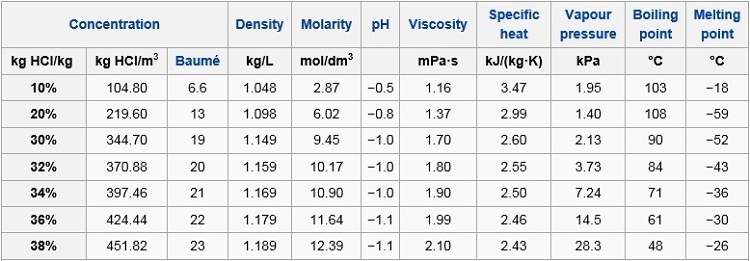
Analytical 4 ACS reagent 2 BioReagent 2 Technique. HYDROCHLORIC ACID is an aqueous solution of hydrogen chloride an acidic gas. This is the students method. The physical properties such as density melting point PH boiling point depends on the molarity or concentration of HCl. Hydrochloric Acid 6M Recommended Use.
 Source: researchgate.net
Source: researchgate.net
The purer the sample the narrower the melting point range. Hydrochloric acid is corrosive to the eyes skin and mucous membranes. Dilution then precipitates the oxychloride BiOCl. By doing this it will also prevent you or someone else cutting. It is the simplest aromatic carboxylic acidThe name is derived from gum benzoin which was for a long time its only sourceBenzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites.
 Source: protank.com
Source: protank.com
Melting point The temperature at which the solidliquid phase change occurs. Metallic bismuth is used principally in alloys to many of which it imparts its own special properties of low melting point and expansion on solidification. At the melting point the solid and liquid phases exist in equilibrium. By doing this it will also prevent you or someone else cutting. Reacts exothermically with organic bases amines amides and inorganic bases oxides and hydroxides of metals.
 Source: sciencedirect.com
Source: sciencedirect.com
An unknown sample of benzoic acid 2-naphthol and naphthalene Table 1 was massed and the unknown number was recorded. The physical properties such as density melting point PH boiling point depends on the molarity or concentration of HCl. Hydrochloric acid is corrosive to the eyes skin and mucous membranes. It is widely used as a laboratory reagent and industry. The melting point is also referred to as liquefaction point solidus or liquidus.

The hydrochloric acid present in the gastric acid helps in the proper digestion of. 1963C 3565F 2236 K Period 5 Boiling point. Preparation of Hydrochloric acid HCl. Benzoic acid b ɛ n ˈ z oʊ. If a spillage occurs during the experiment you should clean it up straight away using a paper towel because it is a safety risk to others as well as yourself.
 Source: msrblog.com
Source: msrblog.com
Experimental Melting Point-1142 C OU Chemical Safety Data No longer updated More details. 1 Structures Expand this section. When contaminated solution Remove. The unknown sample was dissolved in 30mL. This on heating with lime and charcoal produces metallic bismuth.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title hydrochloric acid melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.