Hydrobromic acid boiling point
Home » datasheet » Hydrobromic acid boiling pointHydrobromic acid boiling point
Hydrobromic Acid Boiling Point. P Density g cm 3 0001553. Wt 50F 75F 100F 125F 150F 175F 200F 225F 10C 24C 38C 52C 66C 79C 93C 107C Boiling 25 - - - - - - - - 013 5 - - - - - 001 015 - 078 75 - - - - 001 014 073 - - 10 - - - - 002 051 089 - - 15 - - - 001 034 057 - - - 20 - -. Hydrochloric acid Hydrobromic acid. Chemical Resistance of Rubbers and Elastomers - Rsistance to chemicals.
 Hcl From hydro-land.com
Hcl From hydro-land.com
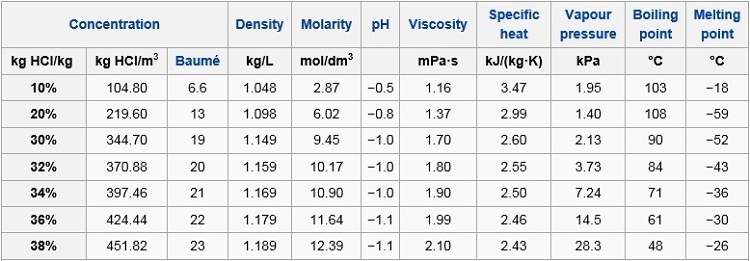
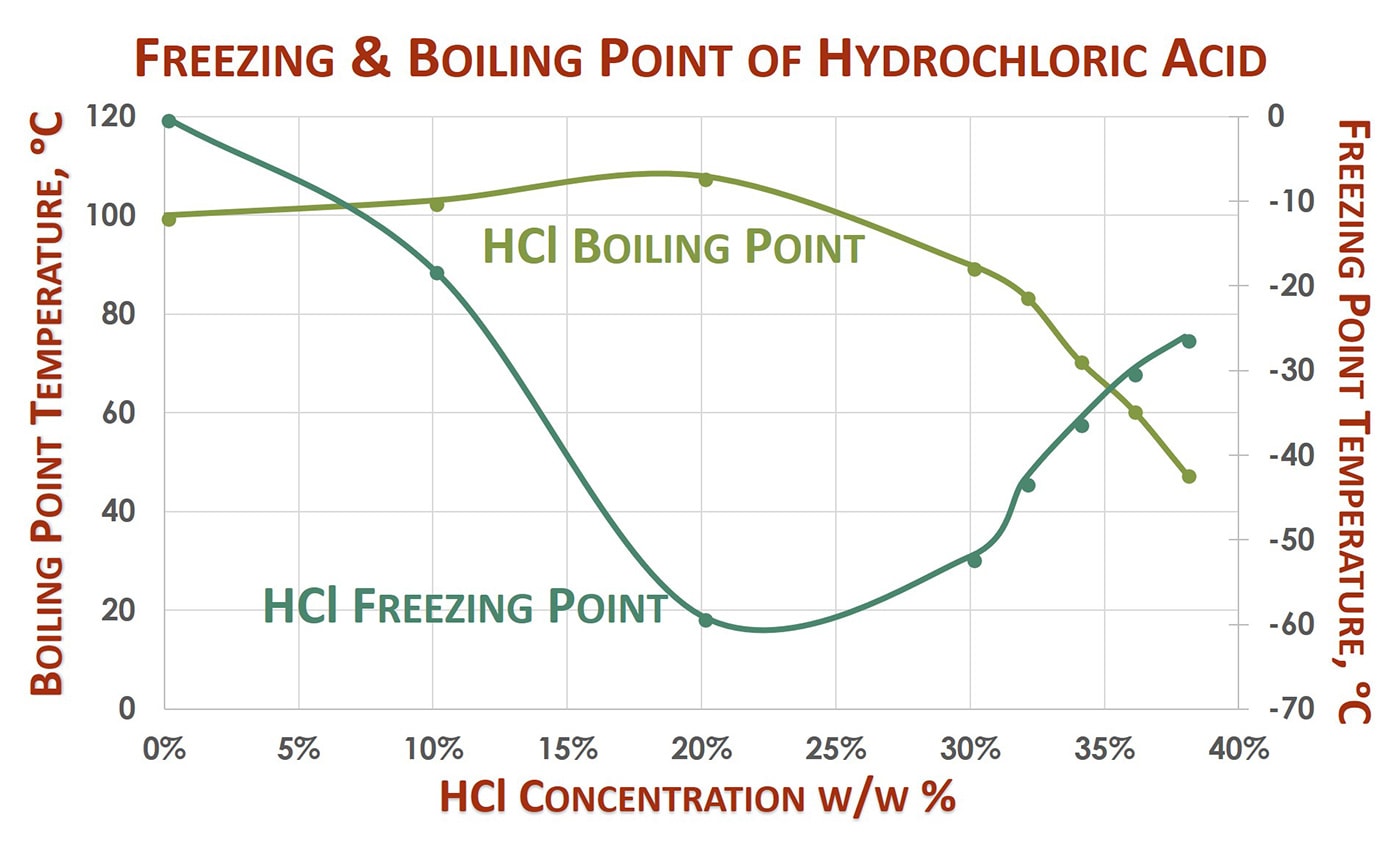
The physical properties such as density melting point PH boiling point depends on the molarity or concentration of HCl. The conclusion that it is impossible to completely convert heat into. 72 C 19 F boiling point. Iodines Aqueous Salt Solution o. 18811C 3066F 8504 K Block. Melting Point C Physical Form.
Ammonium bromide photo grade 9999.
P Density g cm 3 0001553. Constant boiling hydrobromic acid is an aqueous solution that distills at 1243 C and contains 476 HBr by mass which is 877 molL. 18811C 3066F 8504 K Block. Hydrobromic acid is a very solid acid which is formed when hydrogen bromide a diatomic molecule consisting of one hydrogen atom and one bromine atom is dissolved in water. HA H 2 O A-aq H 3 O aq. Corrosion - Corrosion in piping systems - caused by thermodynamic and electrochemical processes - corrosion problems and methods of protection and prevention.
Source: quora.com
Hydroiodic acid or hydriodic acid is an aqueous solution of hydrogen iodide HI. The energy that flows from a warmer body to a colder body is called. Ammonium bromide photo grade 9999. Although both species excrete both the hydroxybutylmercapturic acids only traces of the 2-isomer are excreted by the rabbit. The temperatures are up to the boiling point of the chemical or the melting point of the metal.
 Source: haynesintl.com
Source: haynesintl.com
You will find practical details of a reaction of this kind further down the page. Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More. The 3-isomer has been isolated from rabbit urine as the. Hydroiodic acid or hydriodic acid is an aqueous solution of hydrogen iodide HI. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry.
 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
Preparation of Hydrochloric acid HCl. However this acid is not. Wt 50F 75F 100F 125F 150F 175F 200F 225F 10C 24C 38C 52C 66C 79C 93C 107C Boiling 25 - - - - - - - - 013 5 - - - - - 001 015 - 078 75 - - - - 001 014 073 - - 10 - - - - 002 051 089 - - 15 - - - 001 034 057 - - - 20 - -. Corrosion - Corrosion in piping systems - caused by thermodynamic and electrochemical processes - corrosion problems and methods of protection and prevention. This implies that hydrobromic acid is a stronger acid when compared to hydrochloric acid.
 Source: protank.com
Source: protank.com
Preparation of Hydrochloric acid HCl. The conclusion that it is impossible to completely convert heat into. 400 K 103 bar azeotrope Solubility in water. Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More. 72 C 19 F boiling point.
 Source: chemguide.co.uk
Source: chemguide.co.uk
72 C 19 F boiling point. Thus the material can be used in all strengths of aqueous nitric acid at temperatures up to the boiling point. Entropy of the universe is constantly increasing. 1 1 3 5 7. The conclusion that it is impossible to completely convert heat into.
 Source: hydro-land.com
Source: hydro-land.com
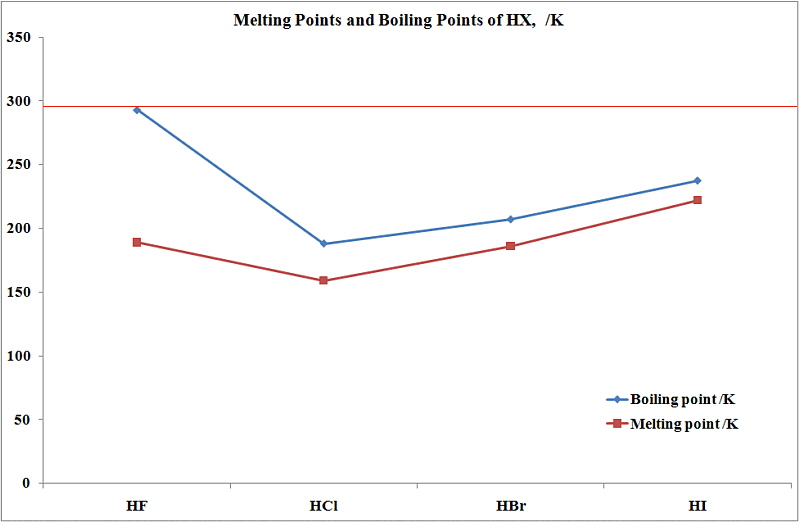
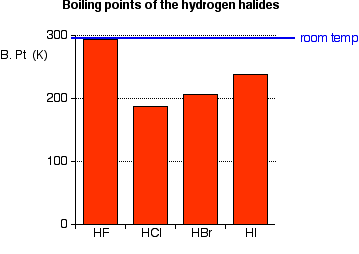
Although both species excrete both the hydroxybutylmercapturic acids only traces of the 2-isomer are excreted by the rabbit. The mixture is warmed to distil off the bromoalkane. Unlike its close relatives hydrochloric and hydrobromic acid HF is a weak acid. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry. There is no evidence of pitting or stress corrosion cracking in aqueous solutions of inorganic metal chlorides.
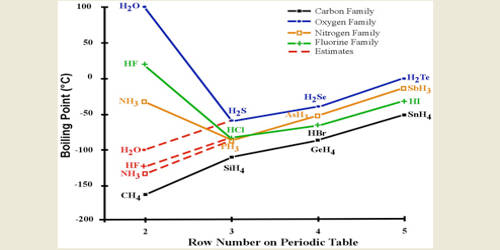 Source: qsstudy.com
Source: qsstudy.com
Ammonium salt of hydrobromic acid. Constant boiling hydrobromic acid is an aqueous solution that distills at 1243 C and contains 476 HBr by mass which is 877 molL. The process by which a solution is heated to its boiling point and the vapors are condensed and collected is known as. Preparation of Hydrochloric acid HCl. You will find practical details of a reaction of this kind further down the page.
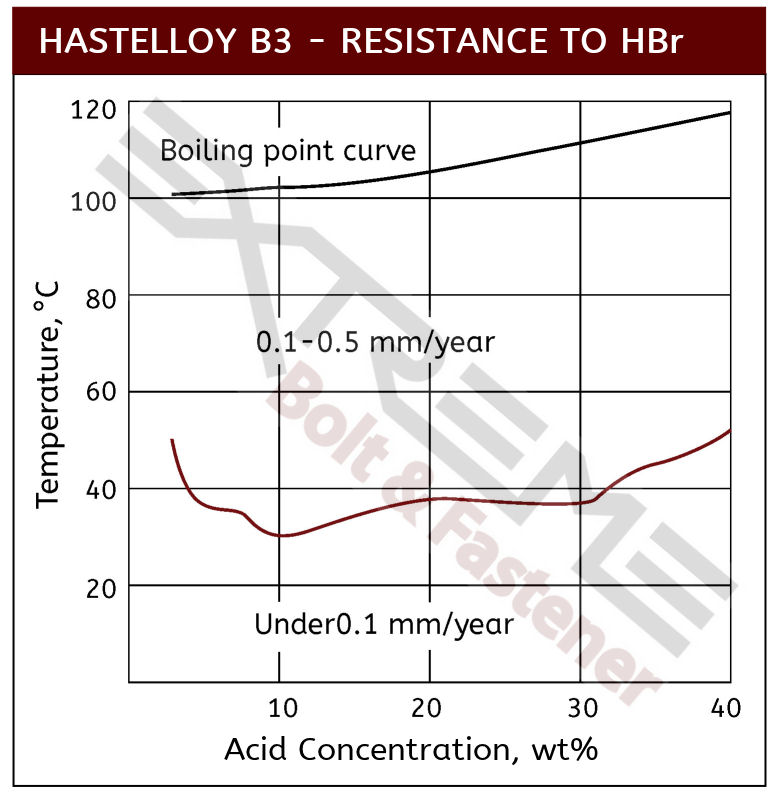 Source: extreme-bolt.com
Source: extreme-bolt.com
Molar mass of naf email protected. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry. In this case the alcohol is. Melting Point C Physical Form. Keyword 7647-01-0 Showing 1-19 of 19 results for 7647-01-0 within Products.
72 C 19 F boiling point. Ammonium bromide photo grade 9999. 59 C 138 F specific gravity. Hydrobromic acid has an acid dissociation constant often denoted by the symbol pKa of magnitude 9. The readiest means of preparing hydrochloric acid gas consists of the application of heat to its strong aqueous solution in other words the spirit of salt or muriatic acid of the shops.

48 wt solution in water Revision Date 25-Apr-2019 Exposure Guidelines 9. 127 C 261 F. Completely Resistant o Moderately Resistant Not Resistant x. Hydrobromic acid has a pK a of 9 making it a stronger acid than hydrochloric acid but not as strong as hydroiodic acid. Chromosulfuric Acid.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title hydrobromic acid boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.