Hf melting point
Home » datasheet » Hf melting pointHf melting point
Hf Melting Point. Answer 1 of 7. Value given for diamond form. Value given for hexagonal gray form. That was Eric Scerri revealing the powers of Hafnium.
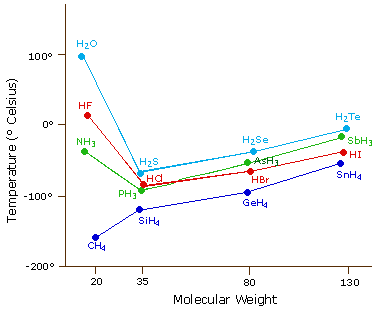
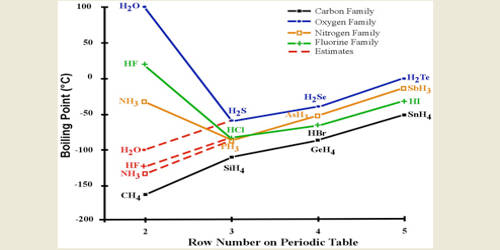 Why Melting And Boiling Points Of Hydrogen Fluoride Is Higher Than Hcl Hbr And Hi Qs Study From qsstudy.com
Why Melting And Boiling Points Of Hydrogen Fluoride Is Higher Than Hcl Hbr And Hi Qs Study From qsstudy.com
Salol has a melting point of about 45C and stearic acid has a melting point of about 69C. The melting point also defines a condition in which the solid and liquid can exist in equilibrium. Now next week we meet the King of the elements. If the pressure is increased to 10 atmospheres carbon graphite is observed to melt at 3550 C. Caesium is a soft silvery-gold alkali metal with a melting point of 285 C which makes it one of only five elemental metals that are liquid at or near room temperature. When considered as the temperature of the reverse change.
Based on the geochemistry and Sr-Nd-Hf-O isotope data the quartz monzonite porphyry with elevated δ 18 O 6674 high εNdt 58 and high εHft 5084 formed by the partial melting of juvenile material ultimately derived from depleted mantle which had been modified by melts derived from sediment on the downgoing slab.
Materials with strong bonds between atoms will have a high melting temperature. That was Eric Scerri revealing the powers of Hafnium. The chemical elements of the periodic chart sorted by. 53 - Covalenz radius. Forget 10 Downing Street or 1600 Pennsylvania Avenue the most prestigious. Below is a table of the melting points boiling points and densities of the elements.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
That was Eric Scerri revealing the powers of Hafnium. Melting Point ºC Boiling Point ºC Density gcm 3 at 293 K 1. They are easily melted in a boiling tube placed in a beaker of hot water. Caesium has physical and chemical properties similar to those of rubidium and potassium. When considered as the temperature of the reverse change.
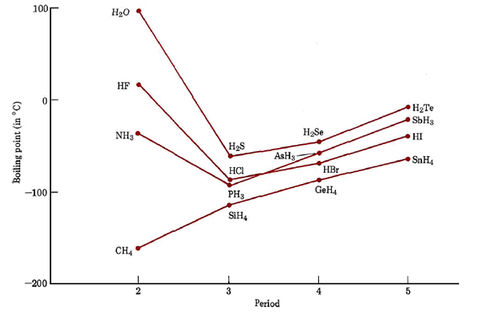 Source: chem.libretexts.org
Source: chem.libretexts.org
If the pressure is increased to 10 atmospheres carbon graphite is observed to melt at 3550 C. 35 - Elements in human body. Value given for alpha form. H 2 TeO 3. Experimental evidence shows that as one moves across the transition metal series in a given period the enthalpy of formation of MB 2 ceramics increases and peaks at Ti Zr and Hf before decaying as the metal gets heavier.
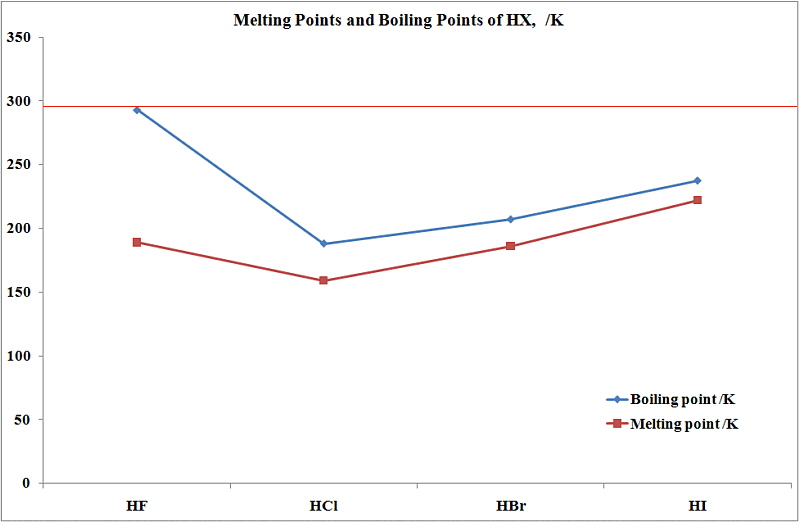 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
35 - Elements in human body. Melting point C. 8 - Boiling point-153. The melting point or rarely liquefaction point of a substance is the temperature at which it changes state from solid to liquid. Caesium has physical and chemical properties similar to those of rubidium and potassium.

H 2 SeO 4. 122 ºC the eutectic point is 82 ºC. Q2 To go. Tungsten rhenium osmium tantalum and molybdenum are among the highest melting point metals. Hg- is the specific enthalpy of the saturated gasvapor under the same listed property values.
 Source: youtube.com
Source: youtube.com
H 2 SO 4. Q2 To go. The melting point of an element is basically the energy required to change the state of an element from its solid state to its liquid state. At the melting point the solid and liquid phase exist in equilibrium. H 3 PO 4.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Garnet is a possible Hf-source since it is a reservoir of Lu which decays to 176 Hf and could be released into the melt during the melting history Tang et al 2014. Below the melting point the solid is the more stable state of the two. H 3 PO 4. Which essentially implies breaking a few bonds. The composition of the liquid would remain at the peritectic point labeled 0 melting until all of the diopside melted.
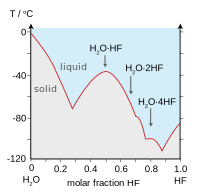 Source: en.wikipedia.org
Source: en.wikipedia.org
If the pressure is increased to 10 atmospheres carbon graphite is observed to melt at 3550 C. 174 Hf 173940 016 20 x 10 15 y. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa. Tungsten rhenium osmium tantalum and molybdenum are among the highest melting point metals. Br 2Br2 59 C and IClICl 97 C Author.

Below the melting point the solid is the more stable state of the two. Barium is a chemical. The lowest mixture melting point e is called the eutectic point. Boiling melting point Viscosity Q1 Rank gas liquid and solid in order of increasing intermolecular forces. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa.
 Source: qsstudy.com
Source: qsstudy.com
85 For chemistry students and. 36 - Vanderwaals radius-108. The temperature can be followed using a thermometer or temperature probe connected to a data logger. 53 - Covalenz radius. 14025 K-258975 C-434 F.
 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
H 3 PO 4. A Periodic table to view elements in 3D. The lowest mixture melting point e is called the eutectic point. The melting point also defines a condition in which the solid and liquid can exist in equilibrium. 2 - Atomic number-259.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title hf melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.