Hexanes boiling point
Home » datasheet » Hexanes boiling pointHexanes boiling point
Hexanes Boiling Point. 69 C 156 F Partition coefficient n-octanolwater. 206 C 403 F. UOP551-86 Hexanes and Lower Boiling Hydrocarbons in Olefin Free Gasolines by GC. To monitor a reactions progress by TLC an aliquot or tiny sample of the reaction mixture is necessaryIf the reaction is run at room temperature or with only mild heating and the concentration of reactants is conducive to TLC a capillary spotter can be directly inserted into the flask where the reaction is taking place Figure 213a.
 Properties Of N Hexane And Methf Download Table From researchgate.net
Properties Of N Hexane And Methf Download Table From researchgate.net
Petroleum ether a petroleum distillation fraction is a mixture of low molecular weight aliphatic hydrocarbons mostly pentanes and hexanes with a low boiling range typically around 30-60oC. UOP555-96 Trace Impurities in High-Purity Benzene and Cyclohexane by GC. Hexanes are chiefly obtained by refining crude oil. Except where otherwise. Take about 10 mg of your crude unknown the tip of a spatula and place in a test tube with about 03 mL of either distilled water hexanes ethyl acetate acetone or ethanol. 353 K Boiling point.
UOP563-90 Packed Apparent Bulk Density of Molecular Sieves.
2340 C 4532 F Evaporation rate. Attach a water-cooled reflux condenser to the round bottomed flask see section above for the proper hook-up of a water-cooled condenser. 95 confidence interval CI for a doubling in PFC concentration. Water has higher molar heat of vaporization than hexane. 2340 C 4532 F Evaporation rate. Easily inhaled or absorbed through the skin hexane has been recognized for more than 40.
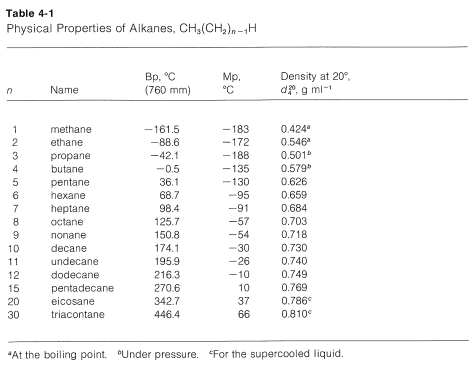 Source: chem.libretexts.org
Source: chem.libretexts.org
Additional Application Specific n-Heptane. 95 CI 078 109 PFNA OR 091. Additional Application Specific Hexanes. UOP563-90 Packed Apparent Bulk Density of Molecular Sieves. Higher concentrations of peroxides.
 Source: researchgate.net
Source: researchgate.net
Heat of evaporation equals about kJmol Any pressure unit can be used. Take about 10 mg of your crude unknown the tip of a spatula and place in a test tube with about 03 mL of either distilled water hexanes ethyl acetate acetone or ethanol. UOP563-90 Packed Apparent Bulk Density of Molecular Sieves. Sequentially add 75 mL diethylamine 25 mL toluene and a few boiling stones. 180 mm Hg at 77 F NTP 1992 Vapor Density Relative to Air.
 Source: thermopedia.com
Source: thermopedia.com
Take about 10 mg of your crude unknown the tip of a spatula and place in a test tube with about 03 mL of either distilled water hexanes ethyl acetate acetone or ethanol. Below enter the boiling point under this pressure C C Above enter a pressure to calculate the boiling point. Water has lower viscosity than hexane. 15 K at atmospheric pressure. If the oil is not subsequently changed the sample may boil when heated in the tube.
Source: chemspider.com
353 K Boiling point. Specific GravityDensity0678 Molecular FormulaC6H14 Molecular Weight8618 Section 10 - Stability and Reactivity Chemical Stability. UOP551-86 Hexanes and Lower Boiling Hydrocarbons in Olefin Free Gasolines by GC. Reacts Solubility soluble in dichloromethane. 206 C 403 F.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Obtaining an Aliquot of a Reaction in Progress. Hexanes are chiefly obtained by refining crude oil. Melting Point-139 F NTP 1992 Vapor Pressure. 180 mm Hg at 77 F NTP 1992 Vapor Density Relative to Air. P260 P280 P284 P305P351P338 P310.
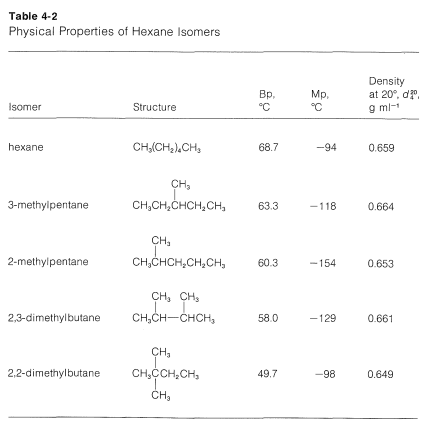 Source: chem.libretexts.org
Source: chem.libretexts.org
Above enter a boiling point to calculate the pressure. Reacts Solubility soluble in dichloromethane. Higher concentrations of peroxides. 62 - 69 deg C 760 mmHg FreezingMelting Point-95 deg C Decomposition TemperatureNot available. UOP563-90 Packed Apparent Bulk Density of Molecular Sieves.
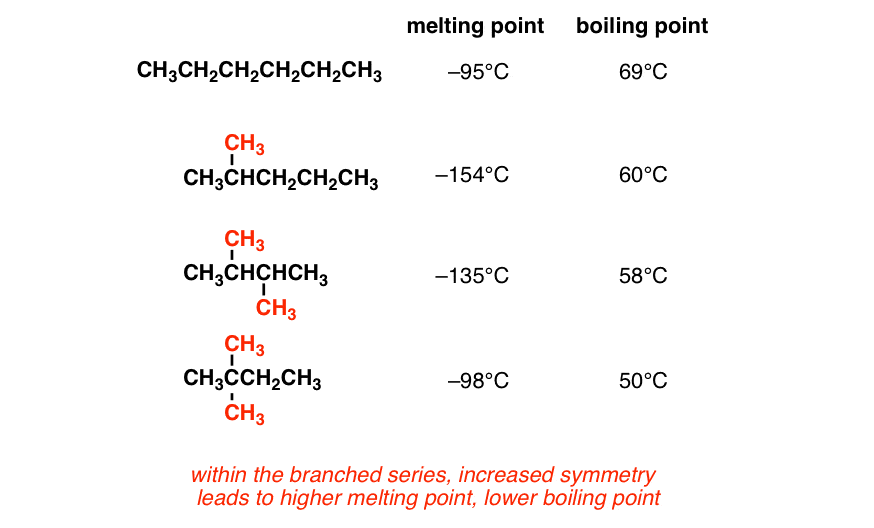 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Stable under normal temperatures and pressures. Articles of petroleum ether are included as well. Hazards Safety data sheet. 2340 C 4532 F Evaporation rate. Not Determined Flash point closed cup.
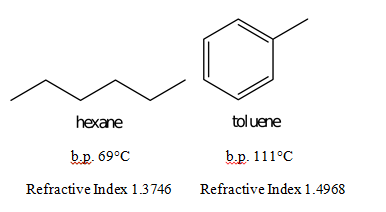 Source: odinity.com
Source: odinity.com
Hexane has a higher vapor pressure than water. C2h2f2 isomers email protected. Heat of evaporation equals about kJmol Any pressure unit can be used. Below enter the boiling point under this pressure C C Above enter a pressure to calculate the boiling point. UOP551-86 Hexanes and Lower Boiling Hydrocarbons in Olefin Free Gasolines by GC.
Obtaining an Aliquot of a Reaction in Progress. Hexane has a higher vapor pressure than water. UOP565-04 Acid Number and Naphthenic Acids by Potentiometric Titration. Attach a water-cooled reflux condenser to the round bottomed flask see section above for the proper hook-up of a water-cooled condenser. 15 K at atmospheric pressure.
 Source: researchgate.net
Source: researchgate.net
80 C 176 F. Reacts Solubility soluble in dichloromethane. Additional Application Specific n-Heptane. For example compounds with a low volatility boiling point 300 C or vapor pressure 01 mm Hg at 20 C are unlikely to create dangerous ie. 206 C 403 F.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title hexanes boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.