Hexane melting point celsius
Home » datasheet » Hexane melting point celsiusHexane melting point celsius
Hexane Melting Point Celsius. -269 C -452 F. 3732 K Boiling point of ethanol. And the same is true for a sample of B containing a little A. Napthalene 100 o C 1248.
 Hexane Data Page Wikipedia From en.wikipedia.org
Hexane Data Page Wikipedia From en.wikipedia.org
Neon -243 o C 540. Mercury 25 o C 1450. The following features will have the effect of creating a higher boiling point. Polypropylene becomes brittle at temperatures below 0 degrees Celsius. The conjugate base formed from the deprotonation of formic acid is commonly referred to as formate. Indium 160 o C 2313.
The lowest mixture melting point e is called the eutectic point.
When any liquid receives enough energy it starts to change its kinetic energy level and go from just a liquid to a liquid and some gas. From lowest point on the graph to highest. Napthalene 100 o C 1248. Email protected email protected. The melting point of commercial isotactic PP varies from 160 to 166 C 320 to 331 F depending on the atactic content and crystallinity. Ethanol also called ethyl alcohol grain alcohol drinking alcohol or simply alcohol is an organic chemical compoundIt is a simple alcohol with the chemical formula C 2 H 6 O.

In 1742 Swedish astronomer Anders Celsius 17011744 created a temperature scale that was the reverse of the scale now known as Celsius. Methanol 20 o C 1116. Hydrocarbons alcohols and acids - boiling points - Boiling temperature C and F with varying carbon number up to C33. It has a lower melting point than NaCl NaCl because the coulombic attractions between its doubly charged Mg2 Mg 2 ions and the S2 S 2 ions are stronger than those between the ions in NaCl NaCl. The melting point of commercial isotactic PP varies from 160 to 166 C 320 to 331 F depending on the atactic content and crystallinity.
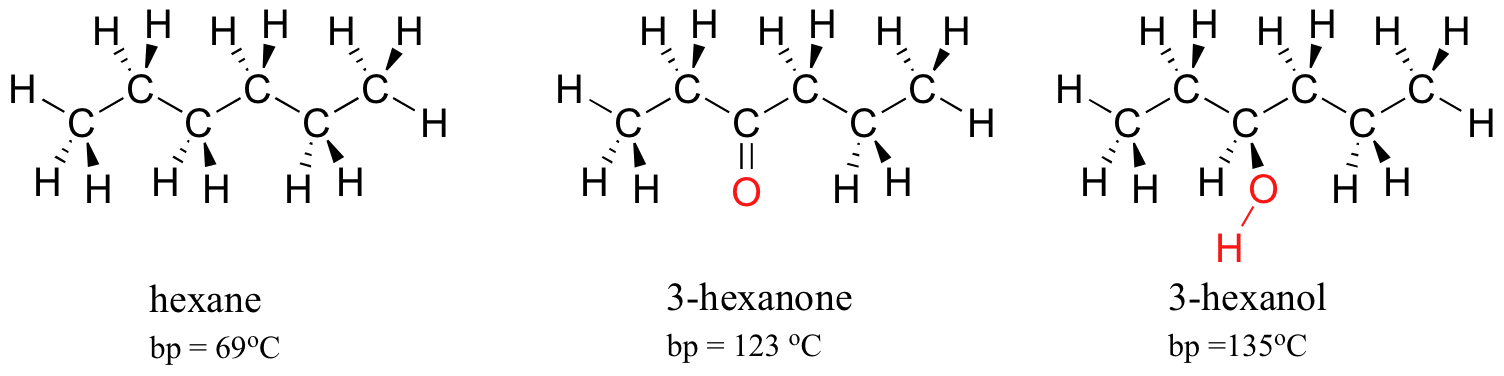 Source: openoregon.pressbooks.pub
Source: openoregon.pressbooks.pub
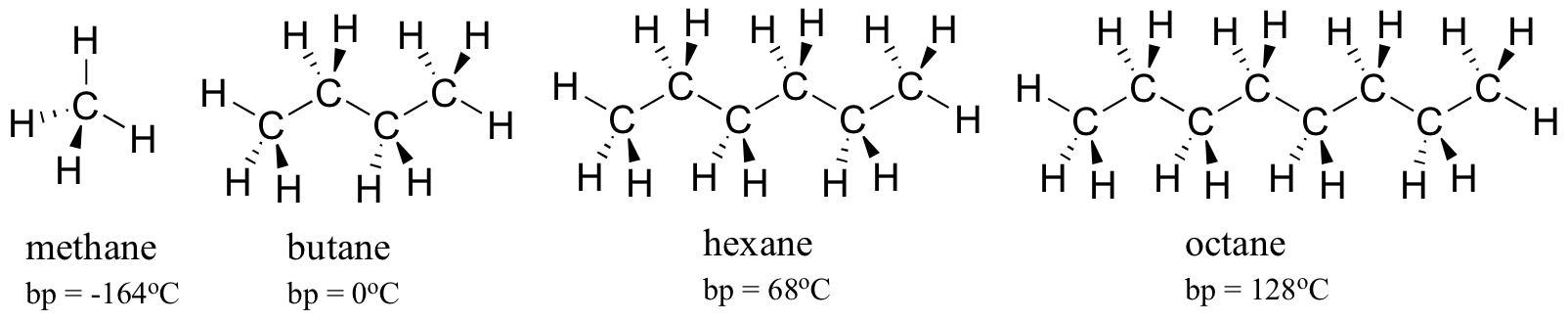
Boiling Points of Alkanes Reminder about Alkanes. Change in FP K f m — assume van t Hoff factor is equal to 1. 137 ºC and B is benzoic acid mp. Please use this book to increase your knowledge for the laboratory pratictioner. Alkanes are chemical compounds that consist only of the elements carbon C and hydrogen H in proportions according to the general formula.
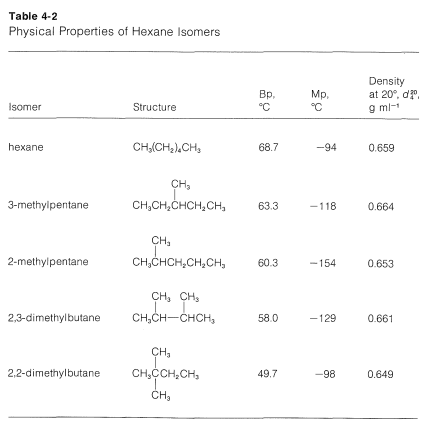 Source: chem.libretexts.org
Source: chem.libretexts.org
Lead 340 o C 1766. The stability of this form is further affected by steric interactions between the hydrogen atoms. The boiling point of butane is close to 0 degrees Celsius whereas the higher boiling point of butanone 796 degrees Celsius can be explained by the shape of the molecule which creates an attractive force between the oxygen on one molecule and the hydrogen on a neighboring molecule. Its formula can be also written as CH 3 CH 2 OH or C 2 H 5 OH an ethyl group linked to a hydroxyl group and is often abbreviated as EtOHEthanol is a volatile flammable colorless liquid with a. In his paper Observations of two persistent degrees on a thermometer he recounted his experiments showing that the melting point of ice is essentially.
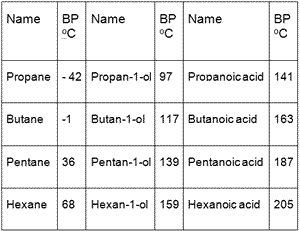 Source: dynamicscience.com.au
Source: dynamicscience.com.au
Answer 1 of 11. PP has a significant thermal expansion but it is slightly less than polyethene. The improve-ments allow new opportunities to optimize the DMTPTA process for maximum purity minimum residue and. The freezing point of a solution prepared by dissolving 150. 100 C 212 F Boiling point of water in Kelvin.
 Source: researchgate.net
Source: researchgate.net
The melting point of syndiotactic PP with a crystallinity of 30 is 130 C 266 F. It can be noted that at a temperature of 25 o Celsius 9999 of the molecules belonging to a given cyclohexane solution would correspond to a chair-type conformation. C n H 2n2 where the letter n represents the number of carbon atoms in each molecule. Methanol 20 o C 1116. Iodobenzne 20 o C 1114.
Hydrocarbons - Physical Data - Molweight melting and boiling point density flash point and autoignition temperature as well as number of carbon and hydrogen atoms in each molecule are given for 200 different hydrocarbons. Iodobenzne 20 o C 1114. It has a lower melting point than NaCl NaCl because the coulombic attractions between its doubly charged Mg2 Mg 2 ions and the S2 S 2 ions are stronger than those between the ions in NaCl NaCl. Oil castor 25 o C 1490. It can either change into a gas when it has reached its boiling point or it evaporates which is just when surface molecules of a liquid get j.
 Source: openoregon.pressbooks.pub
Source: openoregon.pressbooks.pub
C6H14 hexane CS276 C2H4. N-Hexanol 25 o C 1303. This process can be studied by plotting the graph of temperature versus the time during which heat is applied to the system. Nitrobenzene 25 o C 1463. 2 M sucrose or 3 M sucrose.
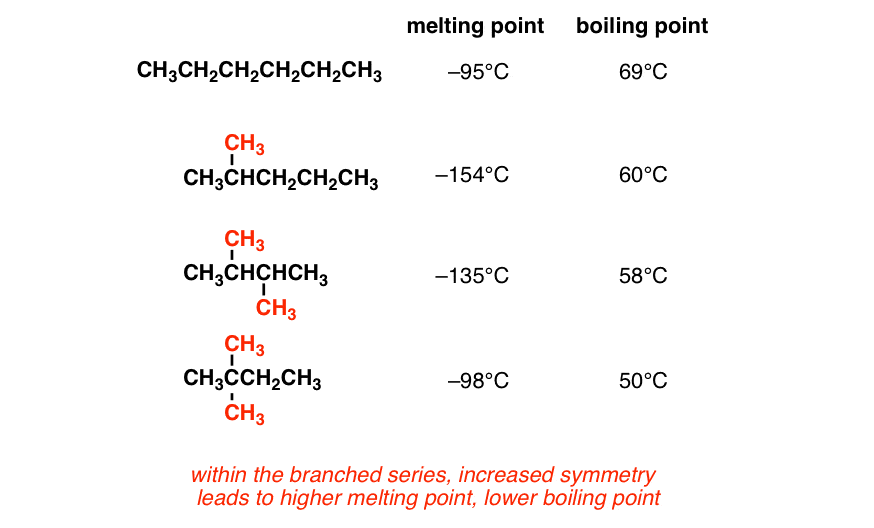 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
In the video you can see that when heat is applied to a solid it is converted into the liquid phase and then into the gas phase. Hydrocarbons alcohols and acids - boiling points - Boiling temperature C and F with varying carbon number up to C33. Email protected email protected. Solid Melting of a solid Liquid Boiling of the liquid gas. Answer 1 of 11.
 Source: wiki.anton-paar.com
Source: wiki.anton-paar.com
C n H 2n2 where the letter n represents the number of carbon atoms in each molecule. In 1742 Swedish astronomer Anders Celsius 17011744 created a temperature scale that was the reverse of the scale now known as Celsius. Must be smoked in its freebase form to experience its effects. The pK a of this weak acid is equal to 3745. It has a lower melting point than NaCl NaCl because the coulombic attractions between its doubly charged Mg2 Mg 2 ions and the S2 S 2 ions are stronger than those between the ions in NaCl NaCl.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It can either change into a gas when it has reached its boiling point or it evaporates which is just when surface molecules of a liquid get j. 137 ºC and B is benzoic acid mp. Neon -243 o C 540. Hydrocarbons alcohols and acids - boiling points - Boiling temperature C and F with varying carbon number up to C33. C6H14 hexane CS276 C2H4.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title hexane melting point celsius by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.