Hexane melting point
Home » datasheet » Hexane melting pointHexane melting point
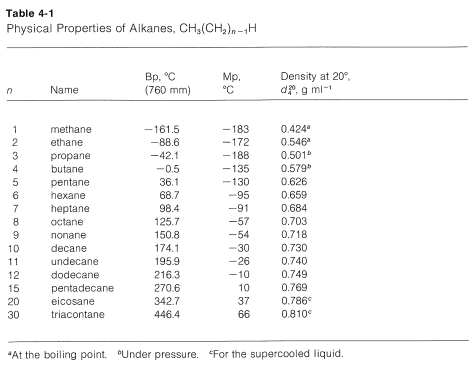
Hexane Melting Point. The boiling point of butane is close to 0 degrees Celsius whereas the higher boiling point of butanone 796 degrees Celsius can be explained by the shape of the molecule which creates an attractive force between the oxygen on one molecule and the hydrogen on a neighboring molecule. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. Fuel Boiling Point o F Acetaldehyde. Stability and Reactivity Stability.
 Properties Of N Hexane And Methf Download Table From researchgate.net
Properties Of N Hexane And Methf Download Table From researchgate.net
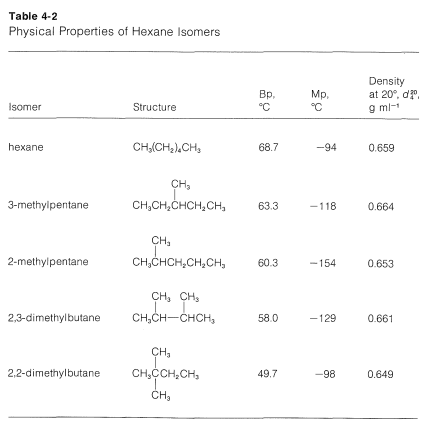
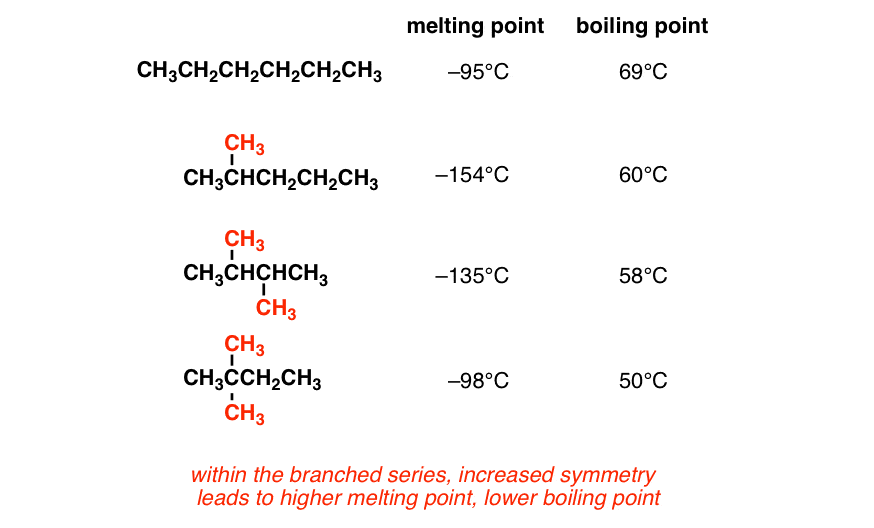
Helium exist as atoms. -1958 C -3204 F Boiling point of liquid helium. The melting point of neopentane 166 C on the other hand is 140 degrees higher than that of isopentane 1599 C and 110 degrees higher than that of n-pentane 1298 C. Stability and Reactivity Stability. The boiling point of a substance is the temperature at which it can change state from a liquid to a gas throughout the bulk of the liquid. The key factor for the boiling point trend in this case is size toluene has one more carbon whereas for the melting point trend shape plays a much more important role.
But this explanation has been challenged on account of it having a lower.
Melting points - freezing points - Documents giving melting or freezing point of elements and different kind of chemical species at varying conditions. Note also that the boiling point for toluene is 111 o C well above the boiling point of benzene 80 o C. Fuel Boiling Point o F Acetaldehyde. It does not form compounds and no intermolecular force between He atoms. 3732 K Boiling point of ethanol. 7C 45F Vapor Density Air1.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Which element has the lowest melting point element in periodic table. Hexane-16-diol HOCH26OH or C6H14O2 CID 12374 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more. Boiling point of water. This makes sense when you consider that melting involves unpacking the molecules from their ordered array.

Page 5 of 7 MSDS Cyclohexane Vapor Pressure mm Hg. The key factor for the boiling point trend in this case is size toluene has one more carbon whereas for the melting point trend shape plays a much more important role. Thermodynamics - Effects of work heat and energy on systems. Melting points - freezing points - Documents giving melting or freezing point of elements and different kind of chemical species at varying conditions. Room temperature is 25 o C Lauric acid which melts at 44 o is still a solid while arachidonic acid has long since melted at.
 Source: researchgate.net
Source: researchgate.net
Hexane ˈ h ɛ k s eɪ n is an organic compound a straight-chain alkane with six carbon atoms and has the molecular formula C 6 H 14. Mercury Hg has the lowest melting point -3883 0 C because mercury has a very weak metallic lattice. 100 C 212 F Boiling point of water in Kelvin. Trans-cyclohexane-12-diol is a cyclohexane-12-diol with trans-configuration. This anomaly has been attributed to the better solid-state packing assumed to be possible with the tetrahedral neopentane molecule.
 Source: thermopedia.com
Source: thermopedia.com
It has a role as a human xenobiotic metabolite. Page 5 of 7 MSDS Cyclohexane Vapor Pressure mm Hg. The boiling point of butane is close to 0 degrees Celsius whereas the higher boiling point of butanone 796 degrees Celsius can be explained by the shape of the molecule which creates an attractive force between the oxygen on one molecule and the hydrogen on a neighboring molecule. This anomaly has been attributed to the better solid-state packing assumed to be possible with the tetrahedral neopentane molecule. -1958 C -3204 F Boiling point of liquid helium.
 Source: chem.libretexts.org
Source: chem.libretexts.org
But this explanation has been challenged on account of it having a lower. Helium exist as atoms. The boiling point of a substance is the temperature at which it can change state from a liquid to a gas throughout the bulk of the liquid. Melting points - freezing points - Documents giving melting or freezing point of elements and different kind of chemical species at varying conditions. It has a role as a human xenobiotic metabolite.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
95 20C 68F Evaporation Rate BuAc1. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. Trans-cyclohexane-12-diol is a cyclohexane-12-diol with trans-configuration. It is a metabolite of cyclohexene oxide and other such compounds. Helium He is the element which has lowest melting point -2722 0 C.
 Source: researchgate.net
Source: researchgate.net
Fuel Boiling Point o F Acetaldehyde. Fuel Boiling Point o F Acetaldehyde. The melting point of neopentane 166 C on the other hand is 140 degrees higher than that of isopentane 1599 C and 110 degrees higher than that of n-pentane 1298 C. 647 C 1485 F Boiling point of acetone. It has a role as a human xenobiotic metabolite.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
7C 45F Vapor Density Air1. 647 C 1485 F Boiling point of acetone. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. 26 Ether 1 10. As the molecular weight increases the melting point increases.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
The boiling point of butane is close to 0 degrees Celsius whereas the higher boiling point of butanone 796 degrees Celsius can be explained by the shape of the molecule which creates an attractive force between the oxygen on one molecule and the hydrogen on a neighboring molecule. Which element has the lowest melting point element in periodic table. 647 C 1485 F Boiling point of acetone. -269 C -452 F. Mercury Hg has the lowest melting point -3883 0 C because mercury has a very weak metallic lattice.
 Source: odinity.com
Source: odinity.com
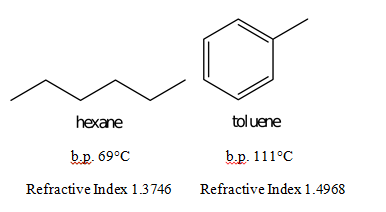
It has a role as a human xenobiotic metabolite. As the molecular weight increases the melting point increases. Hexane-16-diol HOCH26OH or C6H14O2 CID 12374 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Hexane is a significant constituent of gasolineIt is a colorless liquid odorless when pure and with boiling points approximately 69 C 156 F. This anomaly has been attributed to the better solid-state packing assumed to be possible with the tetrahedral neopentane molecule.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title hexane melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.