Hexane melting and boiling point
Home » datasheet » Hexane melting and boiling pointHexane melting and boiling point
Hexane Melting And Boiling Point. 56 C 1328 F Boiling point of alcohol. Chemical Properties of Hexane C 6 H 14. All other group IA materials are solids at room temperature. In this lab we will focus on using Solubility Tests Chemical Tests and Spectra Analysis to identify two unknown compounds.
 Why Do Cyclic Hydrocarbons Have Higher Boiling Points Than Their Acyclic Isomers Chemistry Stack Exchange From chemistry.stackexchange.com
Why Do Cyclic Hydrocarbons Have Higher Boiling Points Than Their Acyclic Isomers Chemistry Stack Exchange From chemistry.stackexchange.com
95 20C 68F Evaporation Rate BuAc1. -1958 C -3204 F Boiling point of liquid helium. Hydrogen exists as a gas at room temperature and francium is a liquid at room temperature. 1 Structures Expand this section. Note also that the boiling point for toluene is 111 o C well above the boiling point of benzene 80 o C. Boiling and melting points.
Lithium has the highest melting and boiling point while hydrogen has the lowest in IA group.
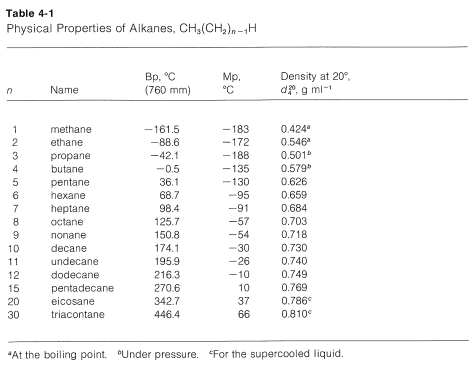
C 6 H 14-95. 7837 C 1731 F Boiling point of methanol. The melting point of neopentane 166 C on the other hand is 140 degrees higher than that of. Mark each of the following statements as TRUE or FALSE. Puren-Hexane is used in laboratories. 4 Spectral Information Expand this section.
 Source: openoregon.pressbooks.pub
Source: openoregon.pressbooks.pub
7837 C 1731 F Boiling point of methanol. The Physical Property fields include properties such as vapor pressure and boiling point as well. This observed in the series lauric C12 palmitic C16 stearic C18. 26 Ether 1 10. Mercury Hg has the lowest melting point -3883 0 C because mercury has a very weak metallic.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Ethanol CH 3CH 2OH mw46 has a boiling point of 78º. A pure substance has the same freezing and melting points in practice a small difference between these quantities can be observed. 2C 6 H 14 19O 2 12CO 2 14H 2 O. The Physical Property fields include properties such as vapor pressure and boiling point as well. C 2 H 6-183-89.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
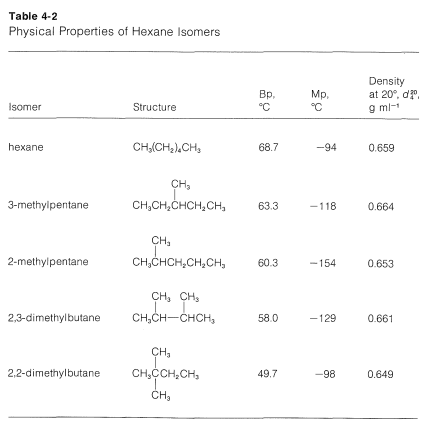
The melting point of neopentane 166 C on the other hand is 140 degrees higher than that of. Hydrocarbons - Physical Data Molweight melting and boiling point density flash. Hexane C 6H 14 mw86 has a boiling point of 68º. Boiling Point Melting Point. Stable at room temperature in sealed containers.
 Source: researchgate.net
Source: researchgate.net
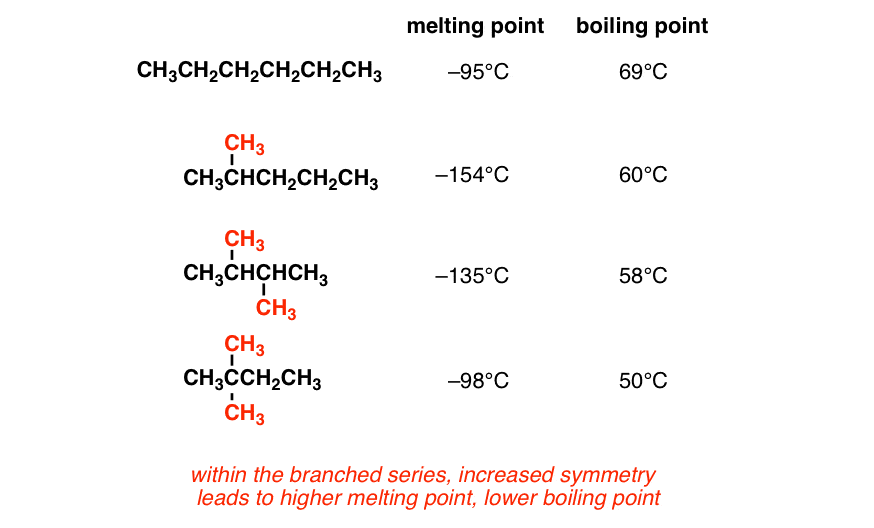
The melting and freezing point changes with pressure but normally they are given at 1 atm. Engineering ToolBox - Resources Tools and Basic Information for Engineering and Design of Technical Applications. The boiling point of neopentane is only 95 C significantly lower than those of isopentane 277 C and normal pentane 360 C. The major use for solvents containing n-Hexane is. Melting Point-139 F NTP 1992.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
C 2 H 6-183-89. -1958 C -3204 F Boiling point of liquid helium. 5 Related Records Expand this. C 3 H 8-190-42. Hexane C 6H 14 mw86 has a boiling point of 68º.
Source: chemspider.com
Boiling point of water. A pure substance has the same freezing and melting points in practice a small difference between these quantities can be observed. The boiling point of neopentane is only 95 C significantly lower than those of isopentane 277 C and normal pentane 360 C. The melting point of a solid compound is the temperature at which a phase transition from solid to liquid occurs. Note also that the boiling point for toluene is 111 o C well above the boiling point of benzene 80 o C.
 Source: chem.libretexts.org
Source: chem.libretexts.org
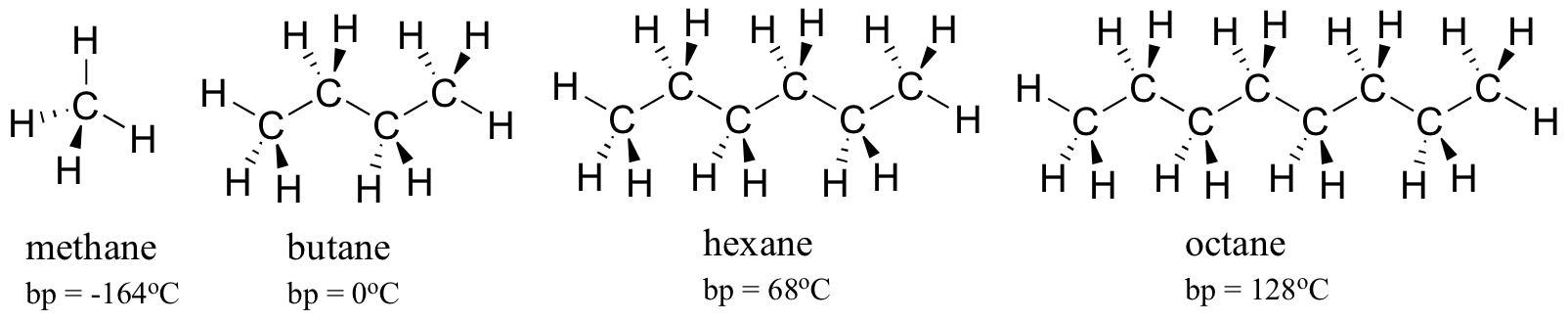
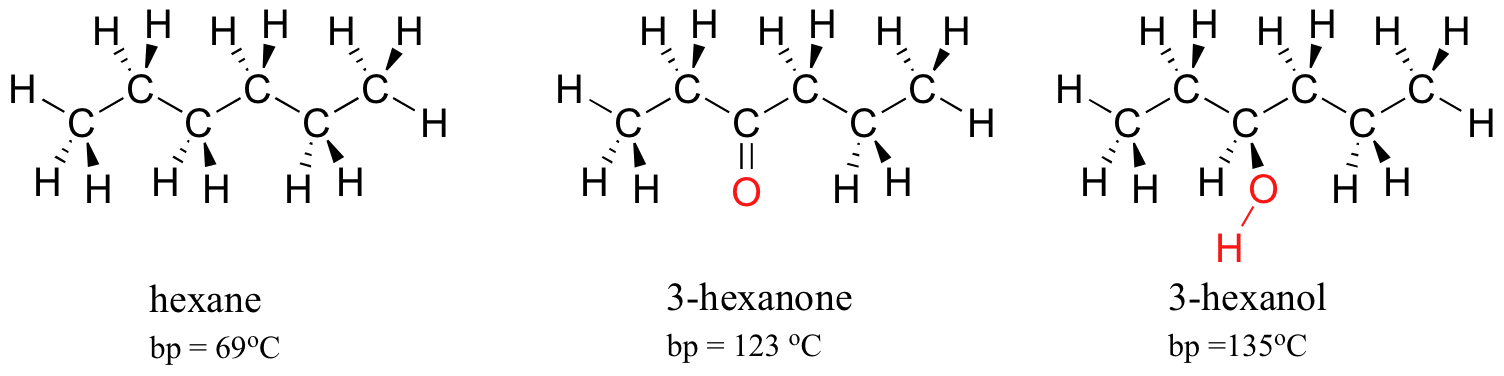
All other group IA materials are solids at room temperature. The boiling point of butane is close to 0 degrees Celsius whereas the higher boiling point of butanone 796 degrees Celsius can be explained by the shape of the molecule which creates an attractive force between the oxygen on one molecule and the hydrogen on a neighboring molecule. Measurement of physical properties includes determining refractive index boiling points melting points and density. Hexane ˈ h ɛ k s eɪ n is an organic compound a straight-chain alkane with six carbon atoms and has the molecular formula C 6 H 14. At the melting point the solid and liquid phases exist in equilibrium.
 Source: openoregon.pressbooks.pub
Source: openoregon.pressbooks.pub
Ethanol has a higher boiling point because of greater London dispersion force c. Freezing point - the temperature at which a liquid turns into a solid. Stability and Reactivity Stability. C 5 H 12-130. C 7 H 16-91.
 Source: thermopedia.com
Source: thermopedia.com
Puren-Hexane is used in laboratories. C 2 H 6-183-89. Lowest melting point from metal elements. A pure substance has the same freezing and melting points in practice a small difference between these quantities can be observed. Hexane ˈ h ɛ k s eɪ n is an organic compound a straight-chain alkane with six carbon atoms and has the molecular formula C 6 H 14.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Chemical Properties of Hexane C 6 H 14. Hexane ˈ h ɛ k s eɪ n is an organic compound a straight-chain alkane with six carbon atoms and has the molecular formula C 6 H 14. 95 20C 68F Evaporation Rate BuAc1. Diethyl ether CH 3 CH 2 2 O. Lowest melting point from metal elements.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title hexane melting and boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.