Hexamethylenediamine melting point
Home » datasheet » Hexamethylenediamine melting pointHexamethylenediamine melting point
Hexamethylenediamine Melting Point. The demand for HDPE resins is mainly associated with blow and injection molding end-uses representing most of the total production. It and nylon 6 are the two most common for textile and plastic industries. Hexamethylene diisocyanate is mainly used to make polyurethane foams and coatings. 1 Upper Explosive Limit.
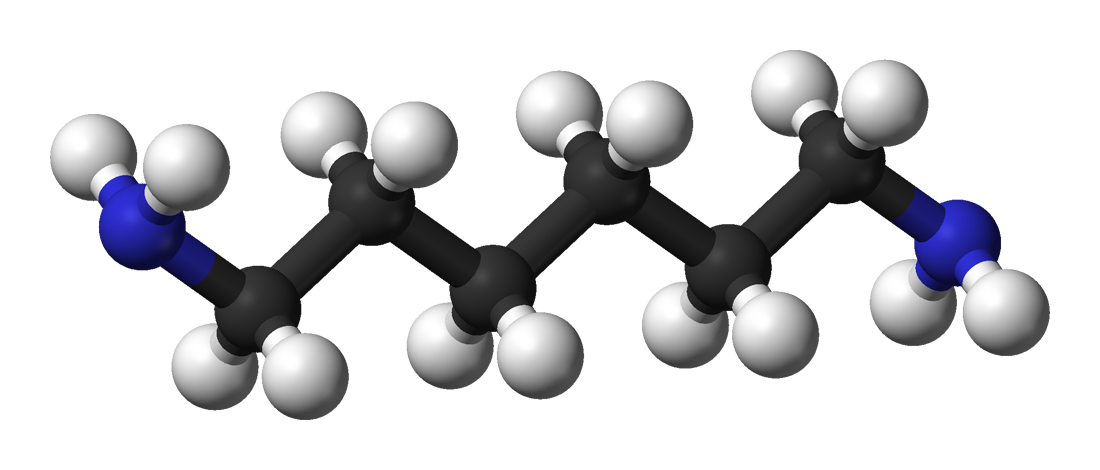 Hexamethylenediamine Wikipedia From en.wikipedia.org
Hexamethylenediamine Wikipedia From en.wikipedia.org
National Toxicology Program Chemical Repository Database. 312 to 315 K Boiling point. P261 P280 P305P351P338 P310. Chemical name of melamine is. HDPE has attractive processing properties such as high chemical stability and low melting point. A dense highly crystalline substance emerges from the presence of a strong intermolecular force.
At room temperature to 910 C it has BCC between 910 C and 1410 C it has face-centered cubic and from 1410 C to its melting point 1539 C it returns to its BCC crystal structure.
Greater than 200 F NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP. Pure iron possesses either BCC or FCC crystal structure as its temperature increases from room temperature to its melting point. This is crystallized to make nylon salt which has precisely stoichiometric equivalents. Nylon 66 is made of two monomers each containing 6 carbon atoms hexamethylenediamine NH_2CH_2_6NH_2. XYRON is an engineering plastic with unique properties created by alloy combinations of PPE with other resins such as polystyrene PS polyamide PA polypropylene PP. The nylon salt goes into a reaction vessel where polymerization process takes place either in batches or continuously.
Source: stenutz.eu
HDPE has attractive processing properties such as high chemical stability and low melting point. In short H 2 NFCOOH F being the fibre part with two different terminal groups viz. Nylon 66 is made of two monomers each. H H N C H 2 5 C O n O H. P261 P280 P305P351P338 P310.
 Source: acs.org
Source: acs.org
Additives and colorings can modify the appearance of the polymer. A Absolute molecular weight b Average molecular weight c Low molecular weight d Absolute melting point 15. Chemical name of melamine is. It is also commonly known as HDI 16-hexamethylene diisocyanate 16-diisocyanatohexane Mondur HX and Desmodur H. Equivalent amounts of hexamethylenediamine and adipic acid are combined with water in a reactor.
 Source: acs.org
Source: acs.org
Research Triangle Park North Carolina. A Absolute molecular weight b Average molecular weight c Low molecular weight d Absolute melting point 15. The density of this engineering plastic is approximately 1314 grams per millilitre. Polymer_handbookpdf - Free ebook download as PDF File pdf Text File txt or read book online for free. Vulcanization is a chemical process in which the rubber is heated with sulphur accelerator and activator at 140160C.
 Source: en.wikipedia.org
Source: en.wikipedia.org
While nylon 6 is produced from caprolactum and has the general formula is. Copolymer of terephthalic acid and isophthalic acid with bisphenol A. The nylon salt goes into a reaction vessel where polymerization process takes place either in batches or continuously. The process involves the formation of cross-links between long rubber molecules so as to achieve improved elasticity resilience tensile strength viscosity hardness and weather resistance. Liquid crystal copolyester with a melting point of not less than 270 ºC whether or not containing fillers.

The density of this engineering plastic is approximately 1314 grams per millilitre. It results in lower melting point boiling point density viscosity dielectric constant and electrical conductivity of ammonia than water. H H N C H 2 6 N H C O C H 2 4 C O n O H. At room temperature to 910 C it has BCC between 910 C and 1410 C it has face-centered cubic and from 1410 C to its melting point 1539 C it returns to its BCC crystal structure. 490 g L 1.
Source: chem.nlm.nih.gov
Also it presents high tensile strength for its weight and is weather resistant. It is presented next. The melting point of high-density polyethylene is relatively high when compared to other polymers. EINECS 240-032-4 and hexamethylenediamine EINECS 204-679-6Polyhexamethylene biguanide monomer. Greater than 200 F NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP.
 Source: fishersci.se
Source: fishersci.se
490 g L 1. Formic acid is the chemical responsible for the horrific pain that occurs due to an ant-bite. 490 g L 1. Therefore it may be processed by many conventional techniques. H H N C H 2 5 C O n O H.
 Source: sciencedirect.com
Source: sciencedirect.com
Nylon 66 is produced through reaction of hexamethylenediamine and adipic acid. Nylon 66 loosely written nylon 6-6 nylon 66 nylon 66 or nylon 66 is a type of polyamide or nylon. And polyamide 66 made from hexamethylenediamine and adipic acid. Polymer_handbookpdf - Free ebook download as PDF File pdf Text File txt or read book online for free. Vulcanization is a chemical process in which the rubber is heated with sulphur accelerator and activator at 140160C.
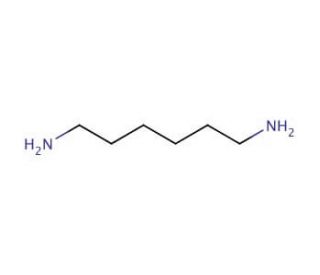 Source: scbt.com
Source: scbt.com
It is also commonly known as HDI 16-hexamethylene diisocyanate 16-diisocyanatohexane Mondur HX and Desmodur H. Within a year Carotherss six researchers had narrowed the field to two possibilities. The melting point of high-density polyethylene is relatively high when compared to other polymers. The key properties exhibited by this polymer are listed below. It is an industrial chemical that is not known to occur naturally.
Source: chemspider.com
Pure iron possesses either BCC or FCC crystal structure as its temperature increases from room temperature to its melting point. Semi-crystalline polycyclohexylenedimethylene terephthalate polymer resin containing by weight 10 or more but not more than 40 of glass fibre in the form of granules or pellets. Manipulating variables in the reactor such as monomer comonomer catalyst and cooling media flowrates can control key quality parameters. A Absolute molecular weight b Average molecular weight c Low molecular weight d Absolute melting point 15. 264 C 507 F Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title hexamethylenediamine melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.