Hcl normal boiling point
Home » datasheet » Hcl normal boiling pointHcl normal boiling point
Hcl Normal Boiling Point. 276 to 280 K Solubility in water. Density 15 C4 C. Data given in this calculator refer to normal pressure that is 101325 hPa. The boiling point is specific for the given substance.
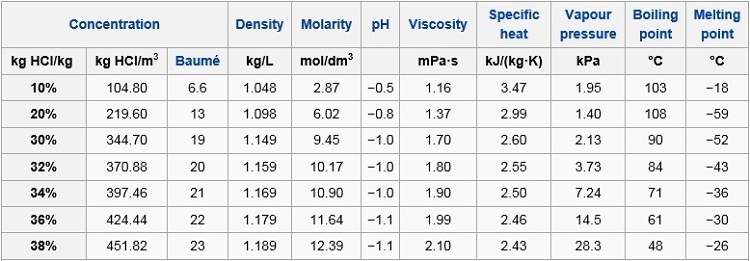 Hcl From hydro-land.com
Hcl From hydro-land.com
1887 kPa at 20 C Henrys law constant k H 95 μmol Pa 1 kg 1. Magnetic susceptibility χ 26010 6 cm 3 mol Refractive index n D 1973 Hazards Safety data sheet. 010 10 N HCl. 2398 K H_2S 34 gmol. Basicity pK b 419 Dipole moment. 373 K HF 20 gmol.
02 mg100 mL Solubility product K sp 143 10 18.
Pyridoxine Hydrochloride is the hydrochloride salt form of pyridoxine a water-soluble vitamin B. 202 001 N HCl. The Normal Saline solution used in medicine for nasal irrigation wound cleaning and intravenous drips is a 091 wv solution of sodium chloride in water. Nothing happens as we slowly compress the container thereby raising the pressure on the gas until the pressure reaches 2 atm. 105 1017 ww solution. The solution will contain 091 g of NaCl in 100 mL of water or 91 g in 1 L.
 Source: slideplayer.com
Source: slideplayer.com
Nothing happens as we slowly compress the container thereby raising the pressure on the gas until the pressure reaches 2 atm. 1 NTP - Normal Temperature and Pressure - is defined as 20 o C 29315 K 68 o F and 1 atm 101325 kNm 2 101325 kPa 147 psia 0 psig 30 in Hg 760 torr 2 STP - Standard Temperature and Pressure - is defined as 0 o C 27315 K 32 o F and 1 atm 101325 kNm 2 101325 kPa 147 psia 0 psig 30 in Hg 760 torr 1 lb m ft 3 16018 kgm 3. 10 years in academic writing. Solution We can solve this problem using four steps. The boiling point is specific for the given substance.

We will guide you on how to place your essay help proofreading and editing your draft fixing the grammar spelling or formatting of your paper easily and cheaply. The Normal Saline solution used in medicine for nasal irrigation wound cleaning and intravenous drips is a 091 wv solution of sodium chloride in water. 656 K sublimes Solubility in water. For example the boiling point of water is 100 C. 33917901 mmHg at 25C Enthalpy of Vaporization.
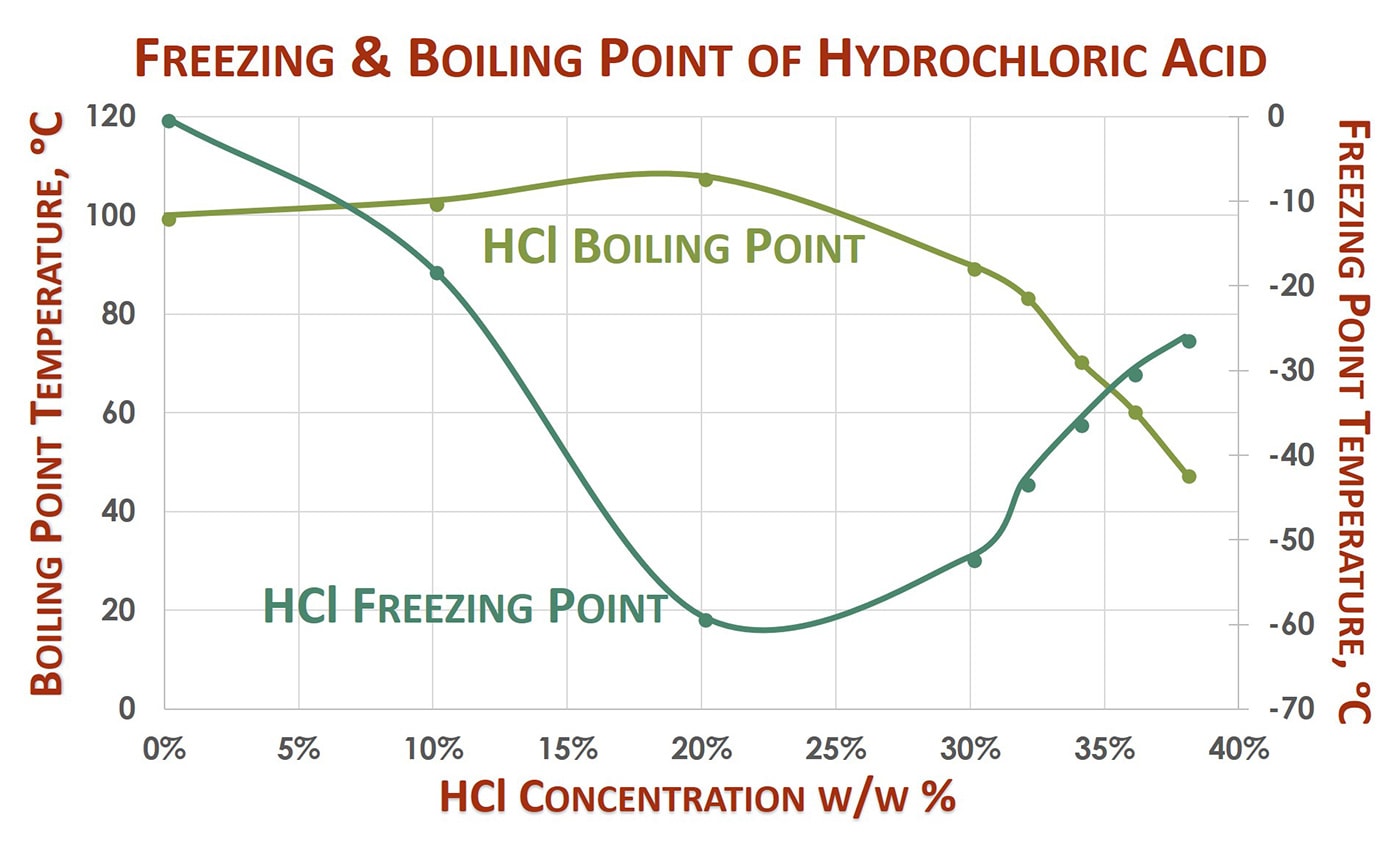 Source: protank.com
Source: protank.com
Basicity pK b 419 Dipole moment. Percent means parts per 100. 2398 K H_2S 34 gmol. A sample of water is heated from a liquid at 40 o C to a gas at 110 o C. 9712 orders delivered before the deadline.
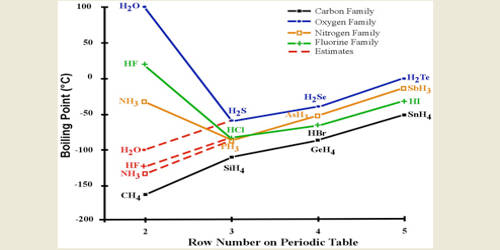 Source: qsstudy.com
Source: qsstudy.com
Since the temperature of the system is above the normal boiling point of water there is no reason for the steam to condense to form a liquid. A On the heating curve diagram provided. For example the boiling point of water is 100 C. Magnetic susceptibility χ 26010 6 cm 3 mol Refractive index n D 1973 Hazards Safety data sheet. -1714 C 1081 solution.
Source: quora.com
Boiling Point-84990 C at 760 mmHg Vapour Pressure. All our clients are privileged to have all their academic papers written. Magnetic susceptibility χ 26010 6 cm 3 mol Refractive index n D 1973 Hazards Safety data sheet. Data given in this calculator refer to normal pressure that is 101325 hPa. 1 liter 1185 gm 444 gm HCl 375 122 moles range 1185 1234 Boiling Point 110C 230F Nitric Acid.
 Source: sciencedirect.com
Source: sciencedirect.com
How would you prepare 15 L of this solution. Cl2 H2O – HOCl HCl OR Cl2 H2O – HOCl H Cl The Hypochlorous Acid ionizes depending on pH to form hydrogen ions H and Hypochlorite ions OCl- and would be expressed as. For example the boiling point of water is 100 C. 1 NTP - Normal Temperature and Pressure - is defined as 20 o C 29315 K 68 o F and 1 atm 101325 kNm 2 101325 kPa 147 psia 0 psig 30 in Hg 760 torr 2 STP - Standard Temperature and Pressure - is defined as 0 o C 27315 K 32 o F and 1 atm 101325 kNm 2 101325 kPa 147 psia 0 psig 30 in Hg 760 torr 1 lb m ft 3 16018 kgm 3. 1 liter 1185 gm 444 gm HCl 375 122 moles range 1185 1234 Boiling Point 110C 230F Nitric Acid.
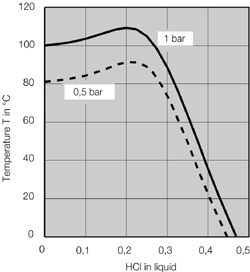 Source: ddpsinc.com
Source: ddpsinc.com
20132015 Morrill Professor Iowa State University. All our academic papers are written from scratch. The solution will contain 091 g of NaCl in 100 mL of water or 91 g in 1 L. Normal reactions 50 - 500 mg of product can be diluted with between 25-100 mL of solvent. Pyridoxine Hydrochloride is the hydrochloride salt form of pyridoxine a water-soluble vitamin B.
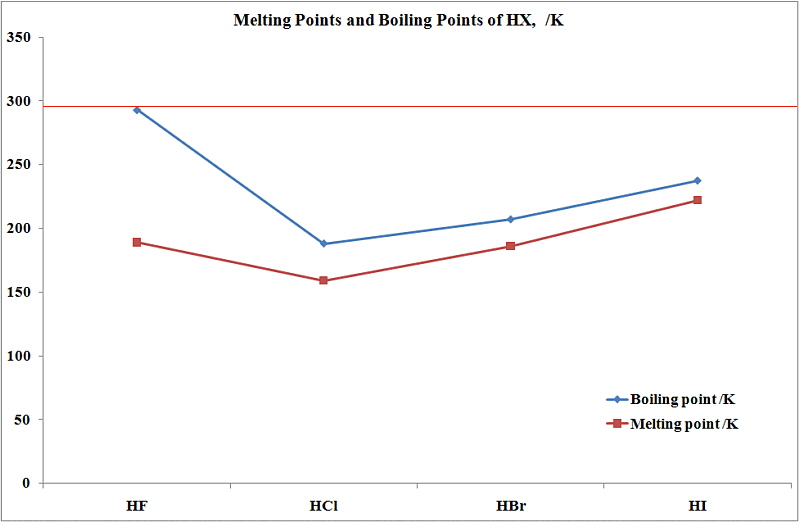 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
The boiling point is specific for the given substance. 9712 orders delivered before the deadline. A solution of hydrogen chloride gas in water. How would you prepare 15 L of this solution. Percent means parts per 100.
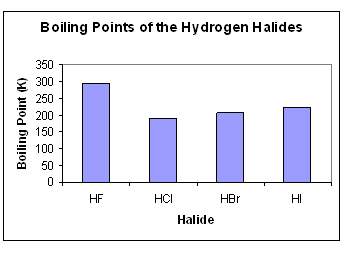 Source: socratic.org
Source: socratic.org
See also Water Boiling points at high pressure Boiling points at vacuum pressure Density specific weight and thermal expansion coefficient Dynamic and kinematic viscosity Enthalpy and entropy Heat of vaporization Ionization Constant pK w of normal and heavy water Melting points at high pressure Saturation pressure Specific gravity Specific heat heat capacity and Specific volume. Data given in this calculator refer to normal pressure that is 101325 hPa. Normal reactions 50 - 500 mg of product can be diluted with between 25-100 mL of solvent. 37 to 44 F. The normal boiling point of a liquid is a the temperature at which the vapor pressure equals 760 torr.
 Source: hydro-land.com
Source: hydro-land.com
Since the temperature of the system is above the normal boiling point of water there is no reason for the steam to condense to form a liquid. 2006 Visiting Professor University of Arizona. May be colored yellow by traces of iron chlorine and organic matter. The boiling point depends on the pressure. 2013-2014 Visiting Lecturer University of Oregon.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title hcl normal boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.