Hbr melting point
Home » datasheet » Hbr melting pointHbr melting point
Hbr Melting Point. Also relative molecular mass 1 is very low. For example the molecule carbon tetrachloride is a non-polar covalent molecule CCl 4. Ethanol must have stronger intermolecular attraction based on its higher boiling point. A bottle of benzene.

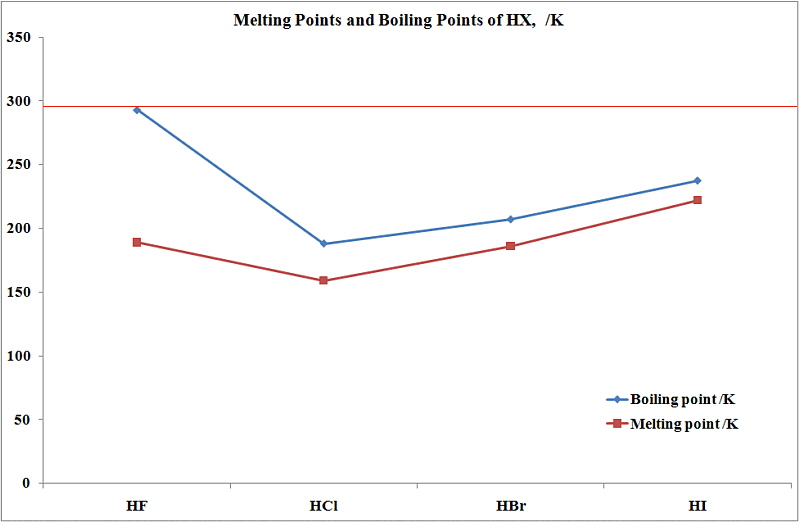
Its melting point is -23C. However its bp189K is lower than that of the less polar HBr 206 K. It does not form compounds and no intermolecular force between He atoms. Helium He is the element which has lowest melting point -2722 0 C. 2 H 2 S H 2 O. The distance between molecules in a crystal lattice is small and regular with intermolecular forces serving to constrain the motion of the molecules more severely than in the liquid state.
GHG emissions are the ideal starting point for such an approach.
DpH does not affect the primary structure of protein pH effects the tertiary structure 16. Free Radical Addition Of HBr To Alkenes With ROOR Peroxides Weve seen that there are three major alkene reactivity patterns carbocation three membered ring and concerted but there are two minor pathways as wellThis post discusses one of them. C the temperature at which the gas molecules have more kinetic energy than the. DpH does not affect the primary structure of protein pH effects the tertiary structure 16. 9712 orders delivered before the deadline. Properties of Covalent Compounds Gases liquids or solids made of molecules Atoms share electrons to become stable.
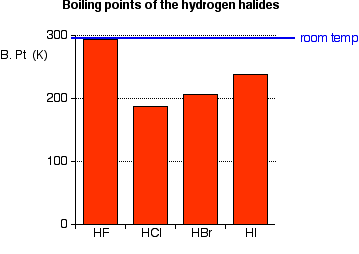 Source: chemguide.co.uk
Source: chemguide.co.uk
Which probably has the lowest boiling point at 100 atm. By contrast the ionic solid NaCl has a melting point of 800C. A HF b HCl c HBr d HI e H 2 SO 4. Which probably has the lowest boiling point at 100 atm. Double and single spacing.
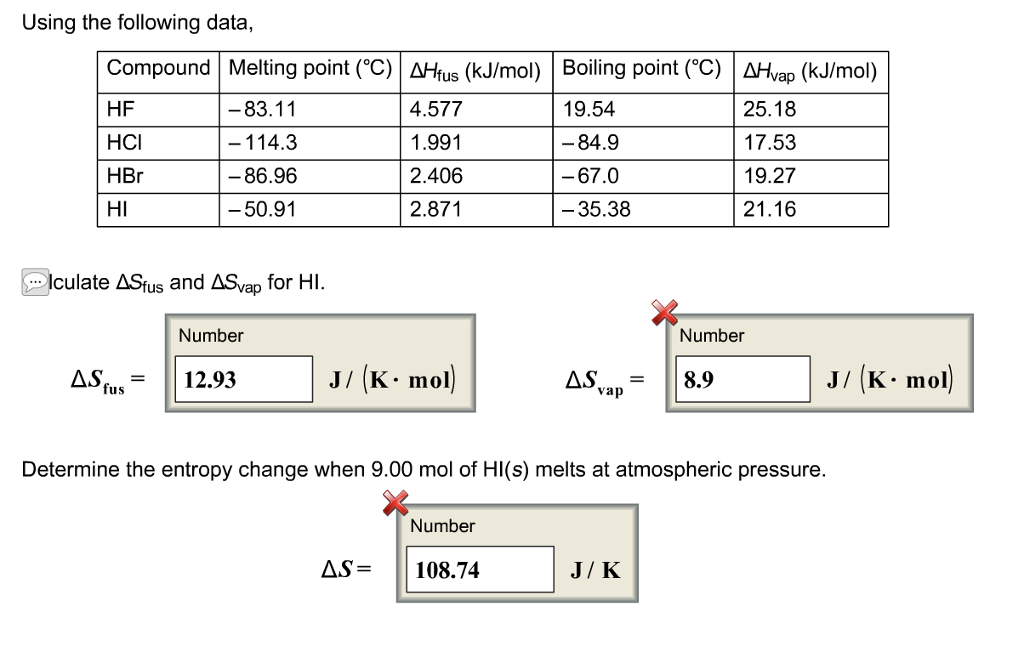 Source: chegg.com
Source: chegg.com
This is due to hydrogen bonding in HF. Bromine was discovered in 1826 by the French chemist Antoine-Jérôme Balard in the residues from the manufacture of sea salt at Montpellier. This is due to hydrogen bonding in HF. Circle all of the species below that can form a hydrogen bond in its pure form. Free-radical addition of HBr to alkenes which shows the opposite regioselectivity anti-Markovnikov than normal addition of HBr to.
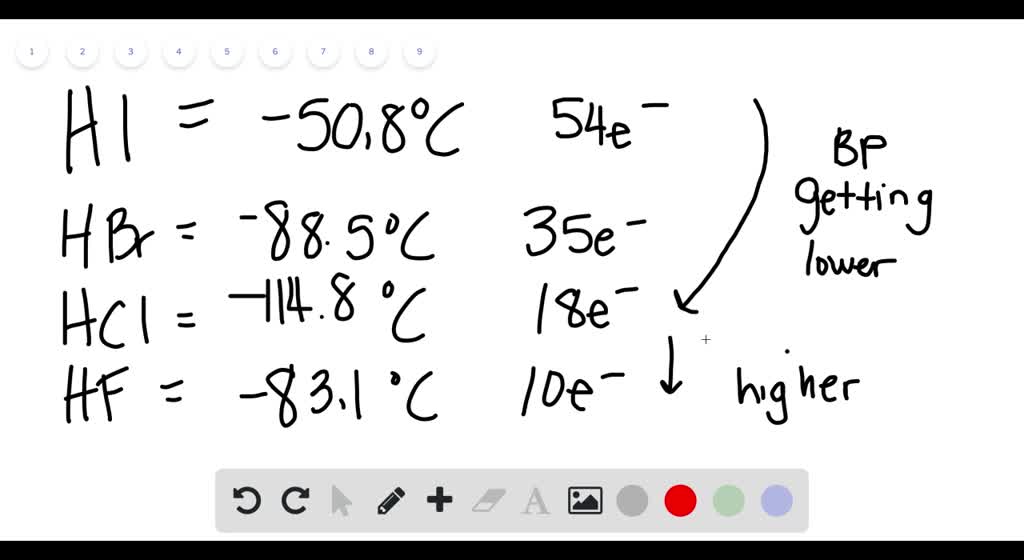 Source: numerade.com
Source: numerade.com
When the temperature reaches 0 o C the melting point of ice further addition of heat does not change the temperature. The melting point is the highest temperature at which crystallization may occur. On the other hand there is a regular decrease in the first ionization energy as we go down this column. 12 point ArialTimes New Roman. From McGraw-Hill Dictionary of Scientific and Technical Terms 4th ed.
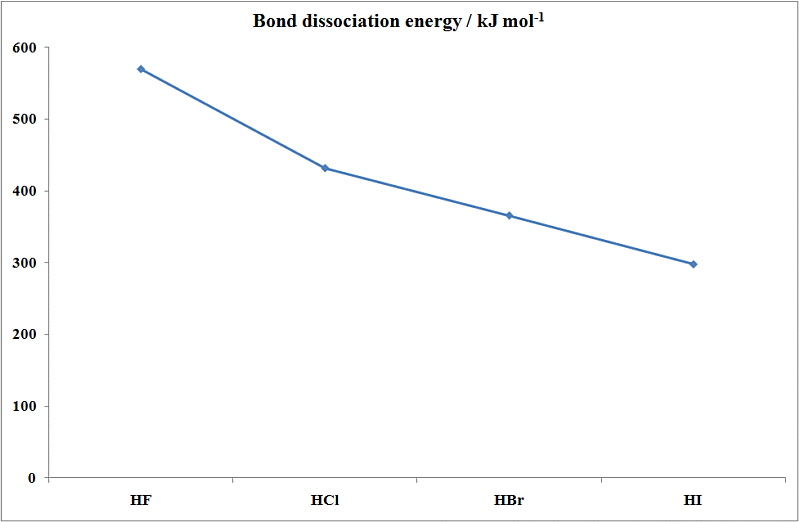 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
Electron configuration Ar3d 10 4s 2 4p 5. Why do compounds having hydrogen bonding have high melting and boiling points. The normal boiling point of a liquid is a the temperature at which the vapor pressure equals 760 torr. All our academic papers are written from scratch. From McGraw-Hill Dictionary of Scientific and Technical Terms 4th ed.
 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
From McGraw-Hill Dictionary of Scientific and Technical Terms 4th ed. 14 mw86 has a boiling point of 68º. Free-radical addition of HBr to alkenes which shows the opposite regioselectivity anti-Markovnikov than normal addition of HBr to. The launch of Pokémon Go this summer was a huge successboth for the gaming industry and for Augmented Reality AR. A HF b HCl c HBr d HI e H 2 SO 4.

2 H 6 CH 3 NH 2 KCl CH 3 CH 2 CH 2 OH CH 3 OCH 3. Mark each of the following statements as TRUE or FALSE. GHG emissions are the ideal starting point for such an approach. Why do compounds having hydrogen bonding have high melting and boiling points. All our academic papers are written from scratch.
 Source: qsstudy.com
Source: qsstudy.com
312 at 20 C 68 F oxidation states. Explain why the other species couldnt hydrogen bond. HBr has 36e-and HCl only 18 e-. The launch of Pokémon Go this summer was a huge successboth for the gaming industry and for Augmented Reality AR. Properties of Covalent Compounds Gases liquids or solids made of molecules Atoms share electrons to become stable.

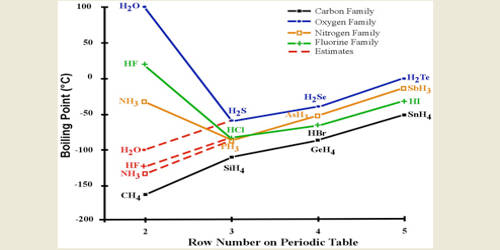
Double and single spacing. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. This is a list showing the boiling points and melting points of saturated and unsaturated hydrocarbons with same number of carbons. 2S boiling point increases down the group but water forms strong hydrogen bonds so has higher boiling point than H 2S 15. All our clients are privileged to have all their academic papers written.

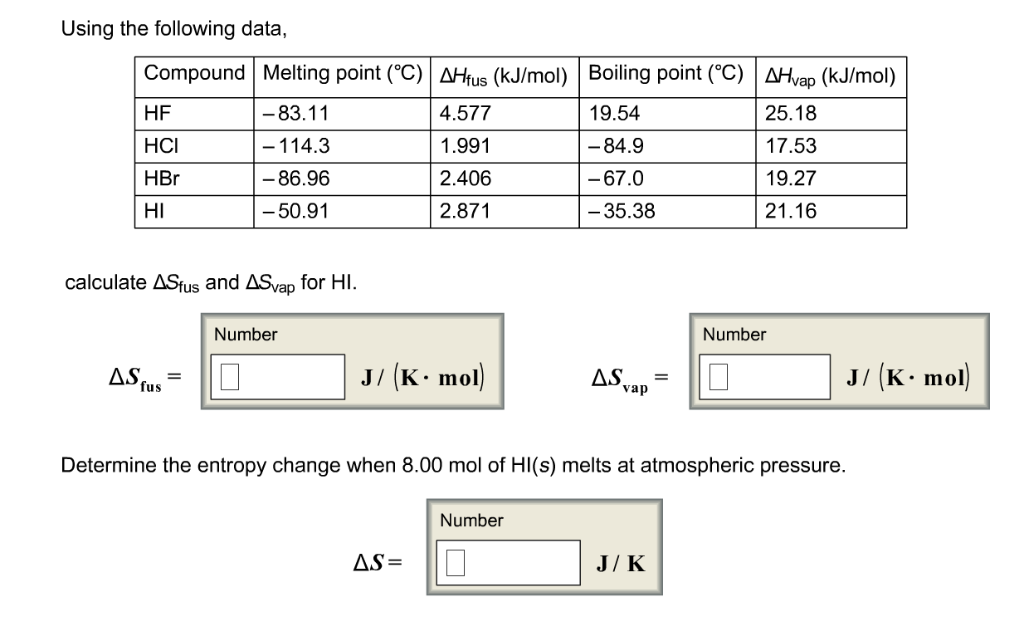
From McGraw-Hill Dictionary of Scientific and Technical Terms 4th ed. A colorless gas it dissolves in water forming hydrobromic acid which is saturated at 6885 HBr by weight at room temperatureAqueous solutions that are 476 HBr by mass form a constant-boiling azeotrope mixture that boils at 1243 C. This is a list showing the boiling points and melting points of saturated and unsaturated hydrocarbons with same number of carbons. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. Substance Formula Melting point C Boiling temperature C Density 25C.
 Source: chegg.com
Source: chegg.com
Double and single spacing. 1 1 3 5 7. Which of the following will have the highest melting point. He liberated the element by passing chlorine through. There is a regular increase in many of the properties of the halogens as we proceed down the column from fluorine to iodine including the melting point boiling point intensity of the color of the halogen the radius of the corresponding halide ion and the density of the element.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title hbr melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.