Hbr boiling point
Home » datasheet » Hbr boiling pointHbr boiling point
Hbr Boiling Point. 8026 8010 016 C. Δt i K b. Hexane C 6H 14 mw86 has a boiling point of 68º. What is the molar mass and molecular formula of the orange compound.

Usually occurs between non-metals. By contrast the ionic solid NaCl has a melting point of 800C. A dipole-dipole interaction is an attraction or repulsion between polar molecules. 1 1 3 5 7. Latest answer posted August 12 2010 at. Thus a melting point reflects the thermal energy needed to convert the highly ordered array of molecules in a crystal lattice to the randomness of a liquid.
A Hydrogen bond is a dipole-dipole interaction.
Boiling point C hydrogen bromide. 1 1 3 5 7. The distance between molecules in a crystal lattice is small and regular with intermolecular forces serving to constrain. Import numpy as np x nplinspace0 nppi 10 print npcosx You can already see from this output that there is a root to the equation cosx 0 because there is a change in sign in the output. Boiling point - the temperature at which a liquid turns into a gas. Order these substances from lowest boiling point to highest.
 Source: api.simply.science
Source: api.simply.science
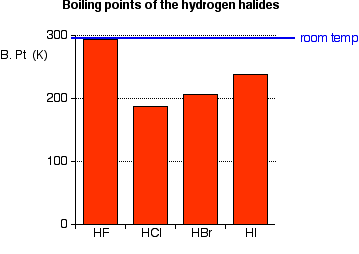
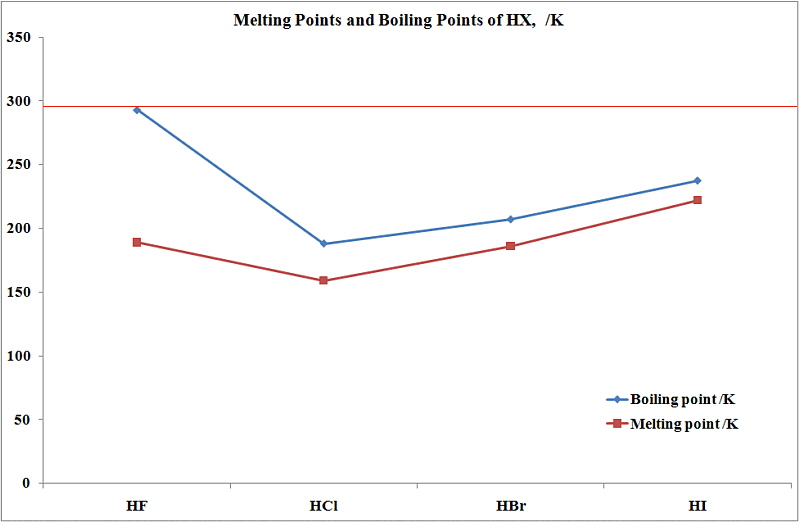
See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Dominant strongest type of IMF for the pure. Electron configuration Ar3d 10 4s 2 4p 5. This explains the non-existence of compounds like KHCl 2 KHBr 2 KHI 2. That HBr has a higher boiling point proves that it is has stronger intermolecular attractions despite its lesser dipole moment.
 Source: chemguide.co.uk
Source: chemguide.co.uk
The CRC Handbook of Chemistry and Physics HBCP contains over 700 tables in over 450 documents which may be divided into several pages all categorised into 17 major subject areas. H 2 SO 4. Mark each of the. H 3 PO 4. Melting point - the temperature at which a solid turns into a liquid.
 Source: snapsolve.com
Source: snapsolve.com
Hydrogen exists as a gas at room temperature and francium is a liquid at room temperature. Boiling point of benzene is 8010 C and ebullioscopic constant of benzene is 253 Cm. Evidently with its extra mass it has much stronger London dispersion attraction enough so to overcome the dipole advantage of HCl. Amy Gallo is a contributing editor at Harvard Business Review co-host of the Women at Work podcast and the author of the HBR Guide to Dealing with. Usually occurs between non-metals.
 Source: qsstudy.com
Source: qsstudy.com
1 1 3 5 7. H 2 SeO 4. Dominant strongest type of IMF for the pure. Low melting and boiling points Poor electrical conductors in all. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.

Thus a melting point reflects the thermal energy needed to convert the highly ordered array of molecules in a crystal lattice to the randomness of a liquid. All other group IA materials are solids at room temperature. Vapor pressure partial pressure of vapor in equilibrium with liquid or solid in a closed container at a fixed temperature. The CRC Handbook of Chemistry and Physics HBCP contains over 700 tables in over 450 documents which may be divided into several pages all categorised into 17 major subject areas. Its melting point is -23C.
 Source: youtube.com
Source: youtube.com
H 3 PO 4. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Mercury Hg has the lowest melting point -3883 0 C because mercury has a very weak metallic lattice. Hydrobromic acid has a pK a of 9 making it a stronger acid than hydrochloric acid but not as strong as hydroiodic acid. Lowest melting point from metal elements.

Read more on Change management or related topics Leadership Psychology and Personal resilience. Usually occurs between non-metals. Δt i K b. Boiling point of benzene is 8010 C and ebullioscopic constant of benzene is 253 Cm. Read more on Change management or related topics Leadership Psychology and Personal resilience.
 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
A Hydrogen bond is a dipole-dipole interaction. B It gives nitroethane on heating with aqueous solution of AgNO 2 C 2H 5Br reacts with metallic Na to give butane gives. What is the molar mass and molecular formula of the orange compound. In the laboratory Heat the flask with a Bunsen burner or electric mantle This causes vapours. Lowest melting point from metal elements.

Vapor pressure partial pressure of vapor in equilibrium with liquid or solid in a closed container at a fixed temperature. All other group IA materials are solids at room temperature. Read more on Change management or related topics Leadership Psychology and Personal resilience. NH3 C2H6 Ne HBr and H2O. H 2 SO 4.

Constant boiling hydrobromic acid is an aqueous solution that distills at 1243 C and contains 476 HBr by mass which is 877 molL. Thus a melting point reflects the thermal energy needed to convert the highly ordered array of molecules in a crystal lattice to the randomness of a liquid. C the temperature at which the gas molecules have more kinetic energy than the. 2 We now utilize this formula. The resulting boiling point is 8026 C.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title hbr boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.