Geh4 boiling point
Home » datasheet » Geh4 boiling pointGeh4 boiling point
Geh4 Boiling Point. The EJ201 and EJ202 engines had multi-point sequential fuel injection and centrally located spark plugs. HClaq hydrochloric acid HFaq hydrofluoric acid H2Saq hydrosulfuric acid Comment. A necessity for chemical engg studs. For example in the ionic compound sodium chloride NaCl the chlorine ion Cl 1 gains one electron that was given by HCl hydrogen chloride HF hydrogen fluoride H2S hydrogen sulfide Rule 2 When dissolved in H2O.
 Relation Intermolecular Forces From 340454897494774470.weebly.com
Relation Intermolecular Forces From 340454897494774470.weebly.com
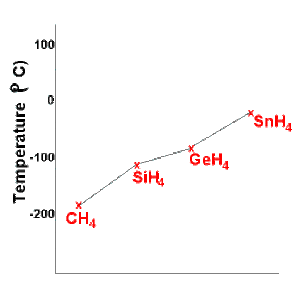
This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. What intermolecular force is responsible. 88 C 126 F. 165 C 265 F. The intermolecular forces responsible for the fact that CH4 has the lowest boiling point in the set CH4 SiH4 GeH4 SnH4 isare _____. Thomas DW et al.
Germanium tetrahydride Germane GeH4 02 06 06 18 Glutaraldehyde OCHCH23CHO - - 02 07 Glycerol mist CH2OHCHOHCH2OH - 10 - - Glycerol trinitrate CH2NO3CHNO3CH2NO3 02 2 02 2 Sk Glycol monoethyl ether C2H5OCH2CH2OH 10 37 02 2 Sk Graphite C - 10 5 - - total inhalable dust respirable dust Guthion CH3O2PSSCH2C7H4N3O - 02 06 - Sk Gypsum CaSO4-2H2O - 10 5 - - total.
What intermolecular force is responsible. Germanium and Germanium Compounds. 1-2 and 3-4 which fired the spark plugs directly twice per cycle. Low Vapor pressure 1 atm. 185 K Solubility in water. 88 C 126 F.
 Source: quizlet.com
Source: quizlet.com
Low Vapor pressure 1 atm. Germanium tetrahydride Germane GeH4 02 06 06 18 Glutaraldehyde OCHCH23CHO - - 02 07 Glycerol mist CH2OHCHOHCH2OH - 10 - - Glycerol trinitrate CH2NO3CHNO3CH2NO3 02 2 02 2 Sk Glycol monoethyl ether C2H5OCH2CH2OH 10 37 02 2 Sk Graphite C - 10 5 - - total inhalable dust respirable dust Guthion CH3O2PSSCH2C7H4N3O - 02 06 - Sk Gypsum CaSO4-2H2O - 10 5 - - total. 108 K Boiling point. 185 K Solubility in water. Thomas DW et al.

Germanium and Germanium Compounds. 1721 μPas theoretical estimate Structure Molecular shape. The intermolecular forces responsible for the fact that CH4 has the lowest boiling point in the set CH4 SiH4 GeH4 SnH4 isare _____. Toxic flammable may ignite spontaneously in air Safety data sheet. The ignition knock control system had fuzzy logic that enabled the maximum ignition advanced angle to be used without detonation.
 Source: slideplayer.com
Source: slideplayer.com
185 K Solubility in water. The EJ201 and EJ202 engines had multi-point sequential fuel injection and centrally located spark plugs. 165 C 265 F. The EJ201 and EJ202 engines had two ignition coils one for each pair of cylinders ie. A necessity for chemical engg studs.
Source: chemguide.co.uk
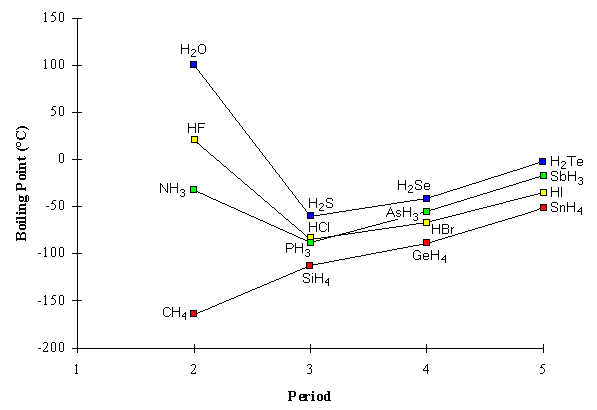
For example in the ionic compound sodium chloride NaCl the chlorine ion Cl 1 gains one electron that was given by HCl hydrogen chloride HF hydrogen fluoride H2S hydrogen sulfide Rule 2 When dissolved in H2O. Covalent bonds have low melting and boiling point. This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. Thomas DW et al. The EJ207 engine had multi-point fuel injection with an injection and firing order of 1-3-2-4.
 Source: vias.org
Source: vias.org
The EJ207 engine had multi-point fuel injection with an injection and firing order of 1-3-2-4. Covalent bonds have low melting and boiling point. Germane GeH4 can also be produced by the reduction of GeCl4 using lithium aluminum hydride or by the reduction of GeO2 by sodium borohydride in water solution. The pentroof combustion chambers had centrally positioned spark plugs and a wide squish area for increased combustion efficiency. Toxic flammable may ignite spontaneously in air Safety data sheet.
 Source: en.wikipedia.org
Source: en.wikipedia.org
1721 μPas theoretical estimate Structure Molecular shape. 185 K Solubility in water. For example in the ionic compound sodium chloride NaCl the chlorine ion Cl 1 gains one electron that was given by HCl hydrogen chloride HF hydrogen fluoride H2S hydrogen sulfide Rule 2 When dissolved in H2O. This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. HClaq hydrochloric acid HFaq hydrofluoric acid H2Saq hydrosulfuric acid Comment.
 Source: legacy.chemgym.net
Source: legacy.chemgym.net
Furthermore the EJ207 engine had a compression ratio of 801. This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. A necessity for chemical engg studs. Furthermore the EJ207 engine had a compression ratio of 801. 165 C 265 F.
Source: quora.com
Article by Ian Lithgow Australian CarReviews Australian CarReviews is an independent publisher. The EJ201 and EJ202 engines had two ignition coils one for each pair of cylinders ie. A Hydrogen bonding B dipole-dipole interactions C London dispersion forces D Mainly hydrogen bonding but also dipole-dipole interactions E Mainly London-dispersion forces but also dipole-dipole interactions. Furthermore the EJ207 engine had a compression ratio of 801. For example in the ionic compound sodium chloride NaCl the chlorine ion Cl 1 gains one electron that was given by HCl hydrogen chloride HF hydrogen fluoride H2S hydrogen sulfide Rule 2 When dissolved in H2O.

Germanium and Germanium Compounds. Covalent bonds have low melting and boiling point. 1-2 and 3-4 which fired the spark plugs directly twice per cycle. 88 C 126 F. Germanium and Germanium Compounds.
 Source: 340454897494774470.weebly.com
Source: 340454897494774470.weebly.com
A Hydrogen bonding B dipole-dipole interactions C London dispersion forces D Mainly hydrogen bonding but also dipole-dipole interactions E Mainly London-dispersion forces but also dipole-dipole interactions. Thomas DW et al. Article by Ian Lithgow Australian CarReviews Australian CarReviews is an independent publisher. Germanium tetrahydride Germane GeH4 02 06 06 18 Glutaraldehyde OCHCH23CHO - - 02 07 Glycerol mist CH2OHCHOHCH2OH - 10 - - Glycerol trinitrate CH2NO3CHNO3CH2NO3 02 2 02 2 Sk Glycol monoethyl ether C2H5OCH2CH2OH 10 37 02 2 Sk Graphite C - 10 5 - - total inhalable dust respirable dust Guthion CH3O2PSSCH2C7H4N3O - 02 06 - Sk Gypsum CaSO4-2H2O - 10 5 - - total. A necessity for chemical engg studs.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title geh4 boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.