Fluorine boiling point
Home » datasheet » Fluorine boiling pointFluorine boiling point
Fluorine Boiling Point. The boiling point of a substance is the temperature at which this phase change boiling or vaporization occurs. 18 Most common isotopes. The boiling point of butane is close to 0 degrees Celsius whereas the higher boiling point of butanone 796 degrees Celsius can be explained by the shape of the molecule which creates an attractive force between the oxygen on one molecule and the hydrogen on a neighboring molecule. Alkali Metals Alkaline Earth Metals Transition Metals Other Metals Metalloids Non-Metals Halogens Noble Gases Rare Earth Elements The halogens are five non-metallic elements found in group 17 of the periodic table.
 Fluorine Uses Properties Facts Britannica From britannica.com
Fluorine Uses Properties Facts Britannica From britannica.com
The boiling point of a substance is the temperature at which this phase change boiling or vaporization occurs. Why zinc has the lowest melting point in 3d metal series. Melting point of Fluorine is -2198C. Energy of first ionisation. A halide is a. Melting and boiling points of 3d metals.
For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. Fluorine also combines with hydrogen to make hydrogen fluoride a colorless gas. Boiling points increases on moving down from fluorine to iodine. -269 C -452 F. Vanadium has the highest melting point and zinc has the lowest melting point. -1958 C -3204 F Boiling point of liquid helium.
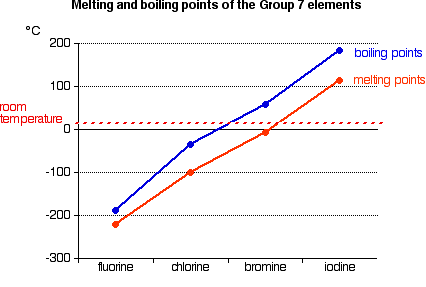 Source: chemguide.co.uk
Source: chemguide.co.uk
Note that these points are associated with the standard atmospheric pressure. 100 C 212 F Boiling point of water in Kelvin. Energy of third ionisation. Fluorine is a chemical element with the symbol F and atomic number 9. Note that these points are associated with the standard atmospheric pressure.
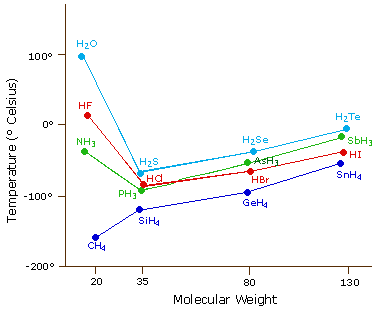 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
The elemenents of the periodic table sorted by boiling point. Boiling point of Fluorine is -1881C. Fluorine is the 13th most abundant element in the crust of the Earth. Through wind-blown soil fluorides are released into the air. For example replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes extending their active lifetimes inside the.
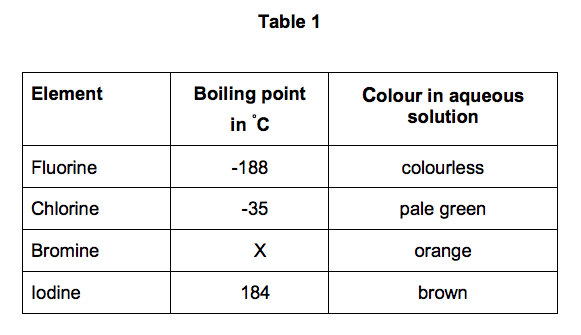 Source: elevise.co.uk
Source: elevise.co.uk
Fluorine is found in nature only in the form of its chemical compounds except for trace amounts of the free element in fluorspar that has been subjected to radiation from radiumNot a rare element it makes up about 0065 percent of Earths crust. All halogens have 7. For example replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes extending their active lifetimes inside the. As can be seen the boiling point of a liquid varies depending upon the surrounding environmental pressure. Boiling point of water.
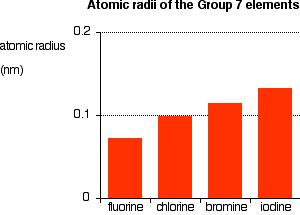 Source: chemguide.co.uk
Source: chemguide.co.uk
The temperature at. For this reason fluorine does not occur free in nature and was extremely difficult for scientists to isolate. Acetic acid anhydride CH 3. Boiling point - the temperature at which a liquid turns into a gas. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure.
 Source: britannica.com
Source: britannica.com
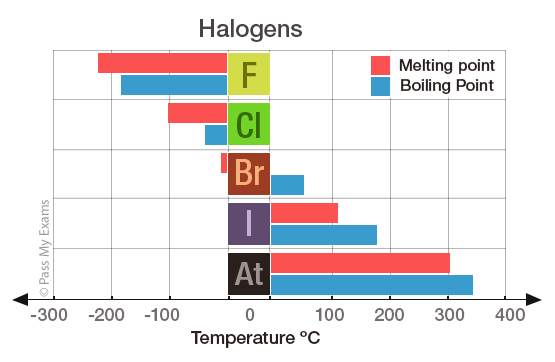
Astatine should have a melting point of about 300C and a boiling point of about 340C. The Earths crust contains 950 parts per million of fluorine. Note that these points are associated with the standard atmospheric pressure. Melting Points of the Halides. For this reason fluorine does not occur free in nature and was extremely difficult for scientists to isolate.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Melting and boiling points of 3d metals are generally higher than s block elements. 2 - Atomic number-253. 56 C 1328 F Boiling point of alcohol. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Electronic shell He 2s 2 2p 5.
 Source: sites.google.com
Source: sites.google.com
Fluorine is the 13th most abundant element in the crust of the Earth. The Earths crust contains 950 parts per million of fluorine. Why zinc has the lowest melting point in 3d metal series. Acetic acid anhydride CH 3. -1958 C -3204 F Boiling point of liquid helium.
 Source: lizzyfluorine.weebly.com
Source: lizzyfluorine.weebly.com
Astatine should have a melting point of about 300C and a boiling point of about 340C. This means that it will be solid at room temperature. Fluorines special status also stems from the fluorine factor the ability of this little atom to fine-tune the chemical properties of an entire molecule. 0136 nm -1. Boiling point of Fluorine is -1881C.
 Source: passmyexams.co.uk
Source: passmyexams.co.uk
The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. A halide is a. 56 C 1328 F Boiling point of alcohol. As the most electronegative element it is extremely reactive as it reacts with all other elements except for argon neon and helium. Melting Points of the Halides.
 Source: rsc.org
Source: rsc.org
Fluorine is found in nature only in the form of its chemical compounds except for trace amounts of the free element in fluorspar that has been subjected to radiation from radiumNot a rare element it makes up about 0065 percent of Earths crust. Atomic number - Name alphabetically-269. All halogens have 7. Through wind-blown soil fluorides are released into the air. Fluorines special status also stems from the fluorine factor the ability of this little atom to fine-tune the chemical properties of an entire molecule.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title fluorine boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.