Ferric chloride boiling point
Home » datasheet » Ferric chloride boiling pointFerric chloride boiling point
Ferric Chloride Boiling Point. Stoichiometry is the chemistry that mathematically relates all substances in a reaction quantitatively relating the amount of reactants and products in a chemical reaction. Kuhn and Barbara A. It allows the chemist to determine the amount of product that will form from a given amount of reactants or the amount of one reactant that is needed to react completely with some specific amount of the other reactant. They are white crystals which do not have an odour but possess a taste.

No data available Flammability solid gas. Hardie Journal of the American Chemical Society 1964 86 6 1055-1060 DOI. At the end of Multiple Choice Questions the answer key has also been provided for. 58 Monday March 26 2012 Rules and Regulations 06142017 EN English US 58 pH. 60 75 Water Evaporation Rate. Properties of Sodium Chloride.
It is slightly soluble in waterIt is noncombustible.
No data available Relative evaporation rate butyl acetate1. Flash point 148C closed cup. Roasting is carried out in 1 A blast furnace 2 A. Ferric chloride can react with metals to form flammable and potentially explosive hydrogen gas. Ag aq Cl aq AgCl s The indicator Fe3 ferric ion is. These values represent the lowest temperatures at which pitting and crevice attack are encountered in this solution within 72 hours.

1015227orgsyn0430015 Since anthracene is significantly. By reflected light the crystals appear dark green but by transmitted light they appear purple-red. 20 Melting Point. It allows the chemist to determine the amount of product that will form from a given amount of reactants or the amount of one reactant that is needed to react completely with some specific amount of the other reactant. Similarly O 2 is the oxide ion Se 2 is the selenide ion and so forth.
 Source: mt.com
Source: mt.com
Then 1mL of. IronIII chloride is the inorganic compound with the formula Fe Cl 3Also called ferric chloride it is a common compound of iron in the 3 oxidation stateThe anhydrous compound is a crystalline solid with a melting point of 3076 C. The ICSC project is a common undertaking between the World Health Organization WHO and. Hardie Journal of the American Chemical Society 1964 86 6 1055-1060 DOI. 2 27 pH solution.

1963 43 15 DOI. It has a melting point of 801C and a boiling point of. The compound is white but typical samples are often off-white. Properties of Sodium Chloride. Ag aq Cl aq AgCl s The indicator Fe3 ferric ion is.
 Source: mistralni.co.uk
Source: mistralni.co.uk
NA Solubility in Water. The main target users are workers and those responsible for occupational safety and health. Ferric chloride is a common compound of iron and chlorine in which iron possesses 3 oxidation state. Uses of Ferric Chloride FeCl 3 Ferric Chloride is used in organic synthesis as a catalyst. Properties of Sodium Chloride.
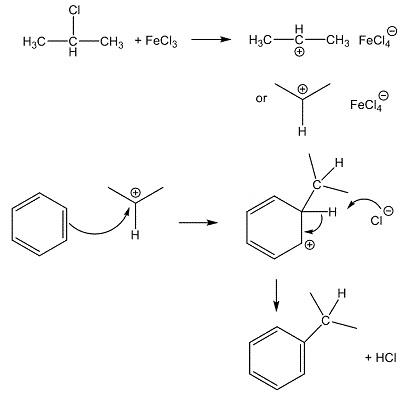 Source: chemicalbook.com
Source: chemicalbook.com
1015227orgsyn0430015 Since anthracene is significantly. The main target users are workers and those responsible for occupational safety and health. No data available Relative evaporation rate butyl acetate1. Roasting is carried out in 1 A blast furnace 2 A. 2 27 pH solution.
 Source: en.wikipedia.org
Source: en.wikipedia.org
FeCl 2 crystallizes from water as the greenish tetrahydrate which is the form that is most commonly encountered in commerce and the laboratory. 1963 43 15 DOI. When wet it is corrosive to aluminum and most metals. For a more detailed explanation check out his article here. It is slightly soluble in waterIt is noncombustible.
 Source: en.wikipedia.org
Source: en.wikipedia.org
These values represent the lowest temperatures at which pitting and crevice attack are encountered in this solution within 72 hours. Boiling Point of Ferric Chloride. 27 Melting point. It is used to treat sewage industrial waste to purify water as. Ferric Chloride and Aluminum Chloride Catalyzed Chlorination of Benzene Alkylbenzenes and Halobenzenes George A.
 Source: chemicalbook.com
Source: chemicalbook.com
Ferric Chloride and Aluminum Chloride Catalyzed Chlorination of Benzene Alkylbenzenes and Halobenzenes George A. Chloride ore among the following is - 1 Malachite 2 Magnesite 3 Magnetite 4 Rock salt. 58 Monday March 26 2012 Rules and. 1963 43 15 DOI. Pick up and remove spilled solid before adding waterIt is used to treat sewage industrial waste to purify water as an etching agent for engraving circuit boards and in the manufacture of other chemicals.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Veteran crystal grower Dmishin found that adding ferric chloride to the salt solution makes the crystals behave better and become more transparent at the cost of yellower crystals. They are white crystals which do not have an odour but possess a taste. No data available Boiling point. The color depends on the viewing angle. For increasing boiling point of metal 3 To make ore porous 4 To remove the impurities 41.
NA Solubility in Water. Flash point 148C closed cup. By reflected light the crystals appear dark green but by transmitted light they appear purple-red. O12 16 Vapor Pressure. These values represent the lowest temperatures at which pitting and crevice attack are encountered in this solution within 72 hours.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title ferric chloride boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.