Ethylbenzene melting point
Home » datasheet » Ethylbenzene melting pointEthylbenzene melting point
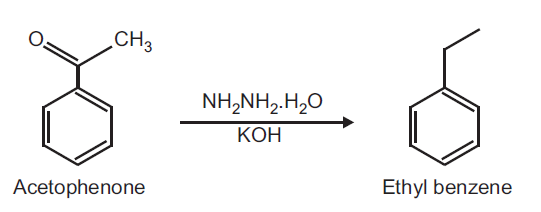
Ethylbenzene Melting Point. Why would benzene have a lower boiling point but a much higher melting point than ethylbenzene. Read More on This Topic. Latent heat of fusion at melting point 11945. 2H319 STOT SE 3H335 STOT RE 2H373 Asp.
 Ethylbenzene 100 41 4 From chemicalbook.com
Ethylbenzene 100 41 4 From chemicalbook.com
Boiling point - 10377 C - 154786 F 16938 K. Benzene has a moderate boiling point and a high melting point. Why would benzene have a lower boiling point but a much higher melting point than ethylbenzene. Extremely Flammable liquid and vapor Harmful if swallowed Skin. Xylenes are released into the atmosphere as fugitive emissions from industrial sources from auto exhaust and through volatilization from their use as solvents. The three isomeric xylenes 12-CH 3 C 6 H 4 CH 3 13-CH 3 C 6 H 4 CH 3 and 14-CH 3 C 6 H 4 CH 3 and another isomer ethylbenzene C 6 H 5 C 2 H 5 can be separated only with difficulty but numerous separation methods have been worked out.
15-130 vol Vapour pressure.
Latent heat of vaporization at boiling point 48241. More than half of the benzene produced each. The oscillating double bonds in the benzene ring are explained with the help of resonance structures as per valence bond theory. Hydrocarbons alcohols and acids - boiling points - Boiling temperature C and F with varying carbon number up to C33. Ethylbenzene 100-41-4 0 5 Cumene 98-82-8 0 5 Methyl Tertiary Butyl Ether MTBE 1634-04-4 0 16 Tertiary Amyl Methyl Ether TAME 994-05-8 0 6. These are chemicals customarily used to kill insects clean toilet bowls strip paint polish brass etch glass preserve corpses and commit murder.
 Source: chemicalbook.com
Source: chemicalbook.com
At one time benzene was obtained almost entirely from coal tar. Melting point - 16915 C - 27247 F 104 K. Benzene has a moderate boiling point and a high melting point. Latent heat of vaporization at boiling point 48241. Benzene has a boiling point of 801 C 1762 F and a melting point of 55 C 419 F and it is freely soluble in organic solvents but only slightly soluble in water.
 Source: en.wikipedia.org
Source: en.wikipedia.org
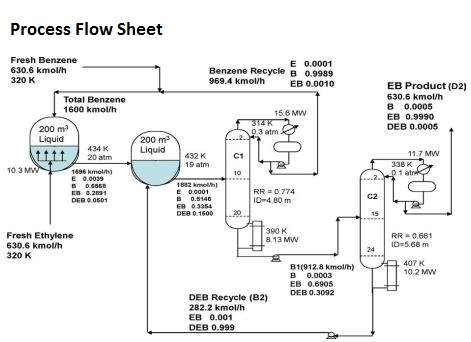
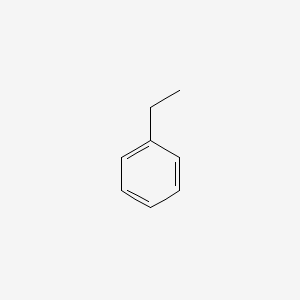
Melting points - freezing points - Documents giving melting or freezing point of elements and different kind of chemical species at varying conditions Related Documents Alcohols and carboxylic acids - physical data - Molweight melting and boiling point density pKa-values as well as number of carbon and hydrogen atoms in each molecule are given for 150 different alcohols and acids. 513887 Btulb 285492 kcalkg. The melting point of ethylene is 1694 C 2729 F and its boiling point is 1039 C 1550 F. Hydrocarbons alcohols and acids - boiling points - Boiling temperature C and F with varying carbon number up to C33. Ethylbenzene is an organic compound with the formula C 6 H 5 CH 2 CH 3It is a highly flammable colorless liquid with an odor similar to that of gasolineThis monocyclic aromatic hydrocarbon is important in the petrochemical industry as an intermediate in the production of styrene the precursor to polystyrene a common plastic material.
 Source: scbt.com
Source: scbt.com
Ethylbenzene is a constituent of petroleum and coal tar and is used as either a petroleum additive or a chemical intermediate in the production of polystyreneHigh level exposure to airborne ethylbenzene is associated with eye and throat irritation. Why would benzene have a lower boiling point but a much higher melting point than ethylbenzene. Acute short- term inhalation exposure to mixed xylenes in humans results in irritation of the eyes nose. Melting point - 16915 C - 27247 F 104 K. It is highly inflammable and burns with a sooty flame.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Ethylene use falls into two main categories. Insoluble in water. Now they are among an estimated 1021 chemicals that the fossil fuel industry mixes with freshwater and forces. Benzene has a boiling point of 801 C 1762 F and a melting point of 55 C 419 F and it is freely soluble in organic solvents but only slightly soluble in water. Ethylene one of the largest volume organic chemicals can be produced either together with acetylene or with propylene.
 Source: chemicalbook.com
Source: chemicalbook.com
2075382 Btulb 1152988 kcalkg. Styrene is the precursor to polystyrene and several copolymers. Hydrocarbons alcohols and acids - boiling points - Boiling temperature C and F with varying carbon number up to C33. The answers are available here for registered members. Insoluble in water.

15-130 vol Vapour pressure. 1H304 5-. August 7 2021 at 318 pm. Ethylbenzene is an organic compound with the formula C 6 H 5 CH 2 CH 3It is a highly flammable colorless liquid with an odor similar to that of gasolineThis monocyclic aromatic hydrocarbon is important in the petrochemical industry as an intermediate in the production of styrene the precursor to polystyrene a common plastic material. Extremely Flammable liquid and vapor Harmful if swallowed Skin.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
64742-95-6 918-668-5 01-2119455851-35-xxxx Hydrocarbons C9 aromatics Flam. In order to determine the nature of an unknown compound correctly other techniques such as NMR 1 H 13 C HETCOR DEPT Mass spectrometry the chemical reactivity solubility tests sometimes derivatives and physical properties melting point boiling point refractive index are required as well. 2075382 Btulb 1152988 kcalkg. Benzene has a boiling point of 801 C 1762 F and a melting point of 55 C 419 F and it is freely soluble in organic solvents but only slightly soluble in water. Latent heat of vaporization at boiling point 48241.
Benzene has a moderate boiling point and a high melting point. Ethylene one of the largest volume organic chemicals can be produced either together with acetylene or with propylene. Which compound has a higher melting point. Acute short- term inhalation exposure to mixed xylenes in humans results in irritation of the eyes nose. 1 as a monomer from which longer.
 Source: tcichemicals.com
Source: tcichemicals.com
It gives rise to. Extremely Flammable liquid and vapor Harmful if swallowed Skin. August 7 2021 at 1154 pm. Ethylbenzene is an aromatic hydrocarbon composed of a benzene ring linked to an ethyl group. At one time benzene was obtained almost entirely from coal tar.

3H226 STOT SE 3H336 5-. Latent heat of fusion at melting point 11945. Ethylene use falls into two main categories. Ethylbenzene is an organic compound with the formula C 6 H 5 CH 2 CH 3It is a highly flammable colorless liquid with an odor similar to that of gasolineThis monocyclic aromatic hydrocarbon is important in the petrochemical industry as an intermediate in the production of styrene the precursor to polystyrene a common plastic material. 513887 Btulb 285492 kcalkg.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title ethylbenzene melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.