Ethylamine boiling point
Home » datasheet » Ethylamine boiling pointEthylamine boiling point
Ethylamine Boiling Point. Only ethylamine and ethanoic acid have the N-H or O-H bonds that are polar enough for hydrogen bonding. Must ALWAYS be stored in approved flammable storage cabinet re. When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils. Ensure that the local ventilation moves the.
Ethylamine C2h7n Chemspider From chemspider.com
A Double the theoretical value b Same as the theoretical value c Half the theoretical value d Three times the theoretical value 12. Hexylamine dipropylamine triethylamine. Metallic form of this metal is soluble in aliphatic amines of a short chain like ethylamine but insoluble in hydrocarbons. Ensure that the local ventilation moves the. Benzaldehyde CAS 100-52-7 o Form. Gardless of amount.
A similar series of reactions has been.
Ensure that the local ventilation moves the. Ethylamine also known as Ethanamine is an organic compound with the formula CH 3 CH 2 NH 2This colourless gas has a strong ammonia-like odorIt condenses just below room temperature to a liquid miscible with virtually all solvents. Liquid colorless bitter almond odor o Use. The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below. C boiling point of p-isomer is more than o- and m-isomer. A Depression in freezing point b Elevation in boiling point c Osmotic pressure d Modification of refractive index 11.
 Source: docbrown.info
Source: docbrown.info
Secondary amines still form hydrogen bonds but having the nitrogen atom in the middle of the chain rather than at the end makes the permanent dipole on the molecule slightly less. Lithium is found only in salts and minerals. Ventilation control of the contaminant as close to its point of generation is both the most economical and safest method to minimize personnel exposure to airborne contaminants. Beer wine and spirits also contain. Liquid colorless bitter almond odor o Use.
 Source: quizlet.com
Source: quizlet.com
Beer wine and spirits also contain. Some liquids and their flash points at atmospheric pressure. Local exhaust ventilation should be applied wherever there is an incidence of point source emissions or dispersion of regulated contaminants in the work area. Acidity pK a 1098 of ammonium form Magnetic susceptibility χ-56810 6 cm 3 mol Refractive index n D 1385 Thermochemistry Heat capacity C 1781 J K 1 mol 1. Acetone CH 3 COCH 3.
 Source: fishersci.fi
Source: fishersci.fi
Some liquids and their flash points at atmospheric pressure. Std enthalpy of. Fuel Flash Point o F Acetaldehyde-36. A Double the theoretical value b Same as the theoretical value c Half the theoretical value d Three times the theoretical value 12. Gardless of amount.
 Source: pinterest.com
Source: pinterest.com
Fuel Flash Point o F Acetaldehyde-36. Gardless of amount. 3279 to 3295 K Solubility in water. Secondary amines still form hydrogen bonds but having the nitrogen atom in the middle of the chain rather than at the end makes the permanent dipole on the molecule slightly less. Boiling point is the temperature at which the saturated vapor pressure equals the external pressure.
242975 kPa Henrys law constant k H 150 μmol Pa 1 kg 1. 41 1D NMR Spectra. Ethylamine also known as Ethanamine is an organic compound with the formula CH 3 CH 2 NH 2This colourless gas has a strong ammonia-like odorIt condenses just below room temperature to a liquid miscible with virtually all solvents. B p-isomer has a symmetrical crystalline structure. Materials with higher flash points are less flammable or hazardous than chemicals with lower flash points.
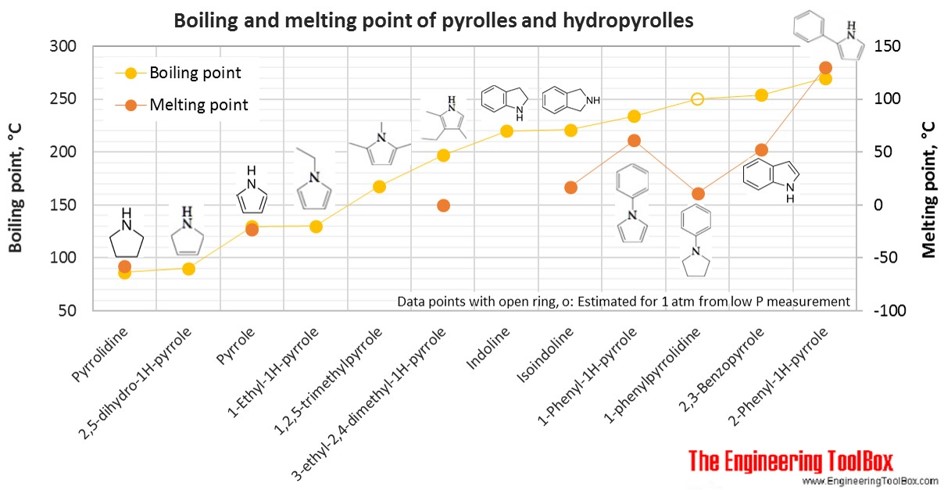 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Acetone CH 3 COCH 3. Liquid colorless bitter almond odor o Use. Precursor for amphetamine or P2P synthesis with nitroethane o Physical properties. 3279 to 3295 K Solubility in water. The one with the greatest potential for hydrogen bonding will have the highest melting point.
 Source: assignmentpoint.com
Source: assignmentpoint.com
Acidity pK a 1098 of ammonium form Magnetic susceptibility χ-56810 6 cm 3 mol Refractive index n D 1385 Thermochemistry Heat capacity C 1781 J K 1 mol 1. When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils. The lithium-ion battery is a key component that is used in many digital devices. Secondary amines still form hydrogen bonds but having the nitrogen atom in the middle of the chain rather than at the end makes the permanent dipole on the molecule slightly less. Flash Boiling Point Point o F o F Limits Density ppm Air 1 Common Name Other Names LEL UEL 1-1 Dichloroethylene Vinylidene chloride 0 99 73 100 34 - Ethylamine.
Source: chemspider.com
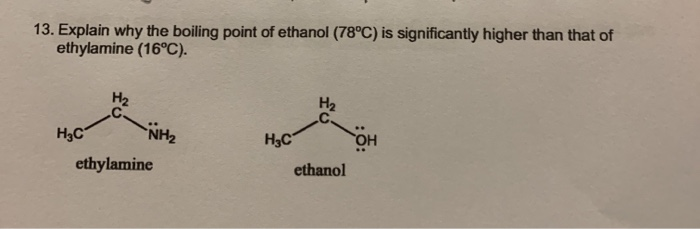
Only ethylamine and ethanoic acid have the N-H or O-H bonds that are polar enough for hydrogen bonding. The one with the greatest potential for hydrogen bonding will have the highest melting point. Flash Boiling Point Point o F o F Limits Density ppm Air 1 Common Name Other Names LEL UEL 1-1 Dichloroethylene Vinylidene chloride 0 99 73 100 34 - Ethylamine. Std enthalpy of. Lithium is found only in salts and minerals.
 Source: chegg.com
Source: chegg.com
Acetic acid anhydride CH 3 COO 2 O. See also Autoignition temperature and flash point of different hydrocarbons. Alcohol - ethyl grain ethanol C. Boiling point 176C 354F vapor pressure 1 mmHg at 26. A similar series of reactions has been.
 Source: merckmillipore.com
Source: merckmillipore.com
Gardless of amount. It also finds use in the manufacture of denatured alcohol in pharmaceuticals and cosmetics lotions perfumes as a chemical intermediate and as a fuel either alone or in mixtures with gasoline. A similar series of reactions has been. C boiling point of p-isomer is more than o- and m-isomer. The molecular weight of sodium chloride determined by measuring the osmotic pressure of its aqueous solution is.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title ethylamine boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.