Ethyl acetate freezing point
Home » datasheet » Ethyl acetate freezing pointEthyl acetate freezing point
Ethyl Acetate Freezing Point. Surface tension 240 mNm at 200 C 680 F Boiling pointBoiling range. Page 6 of 15 MSDS. Ethyl Acetate 99 751 0902 755-780 168-172 24 41 Isopropyl Acetate 99 727 0872 85-90 185-194 42 30 n-Propyl Acetate 739 0888 99-103 210-217 55 23 Isobutyl Acetate 725 0870 112-119 234-246 63 16 n-Butyl Acetate 99 735 0882 120-128 248-262 81 10 Glycol Ether PM Acetate 806 0970 140-150 284-302 114 04 Amyl Acetate primary 729 0876 142-152 288-306 101 049 Isobutyl. Absorption and elimination of dermally applied doses of 14C-diethylene glycol butyl ether and 14C-diethylene glycol butyl ether acetate derivative were determined in Sprague-Dawley ratsThe materials were applied under occlusion for 24 hr at dose levels of 02 and 20 gkg undiluted and as a 10 aqueous solution 02 gkg diethylene glycol butyl ether.
 The Boiling Points Of Ethyl Acetate And Ch 3 Oh Respectively Are 77 C And 65 C Youtube From youtube.com
The Boiling Points Of Ethyl Acetate And Ch 3 Oh Respectively Are 77 C And 65 C Youtube From youtube.com
The problems related to conventional freezing like non-uniform crystal development destruction of food material structure and loss in sensory food quality have given rise to use some innovative technologies such as air blast plate contact fluidized-bed freezing immersion freezing cryogenic freezing high-pressure freezing and their combinations are the most common methods. Specific GravityDensity100 Molecular FormulaH20 Molecular Weight2014. Chemical or scientific names are used to give an accurate description of a substances composition. A ternary mixture of ethyl acetate water and ethyl alcohol distils first. 09006 gmL 7516 lbgal at 20C. The condensation point of water is the same as the boiling point of water.
2-ethylhexan-1-ol is a primary alcohol that is hexan-1-ol substituted by an ethyl group at position 2.
Evaporation Rate1 Butyl acetate1 Viscosity. Absorption and elimination of dermally applied doses of 14C-diethylene glycol butyl ether and 14C-diethylene glycol butyl ether acetate derivative were determined in Sprague-Dawley ratsThe materials were applied under occlusion for 24 hr at dose levels of 02 and 20 gkg undiluted and as a 10 aqueous solution 02 gkg diethylene glycol butyl ether. Ethyl Acetate Solvent Properties. Commercially ethyl acetate is made from 10 acetic acid quick vinegar process and 50 alcohol high wines by a distillation process. The pressure exerted by the vapor phase is called the. -84 C -119 F Solubilities.
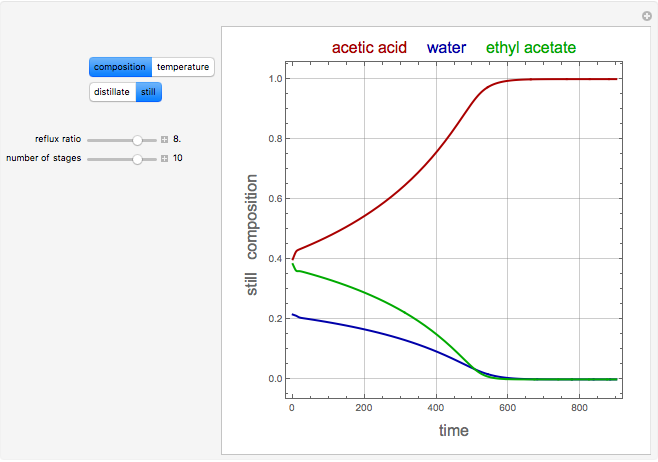 Source: demonstrations.wolfram.com
Source: demonstrations.wolfram.com
It has a role as a volatile oil component and a plant metabolite. Examples include adding salt into water used in ice cream makers and for de-icing roads alcohol in water ethylene or propylene glycol in water used in antifreeze in cars adding copper to molten silver used to make solder that. METHYL ETHYL KETONE TWA 590 mgm3 200 ppm NA OSHA Z1 METHYL ETHYL KETONE STEL 300 ppm NA ACGIH METHYL ETHYL KETONE TWA 200 ppm NA ACGIH. -84 C -119 F Solubilities. Specific GravityDensity100 Molecular FormulaH20 Molecular Weight2014.
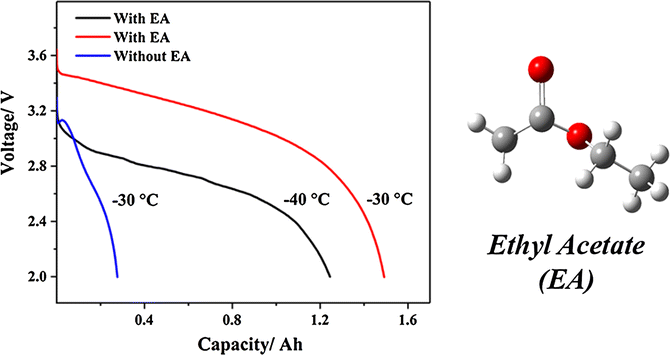 Source: link.springer.com
Source: link.springer.com
Surfactants and 2 freezing point depressants antifreezes. The condensation point of water is the same as the boiling point of water. Absorption and elimination of dermally applied doses of 14C-diethylene glycol butyl ether and 14C-diethylene glycol butyl ether acetate derivative were determined in Sprague-Dawley ratsThe materials were applied under occlusion for 24 hr at dose levels of 02 and 20 gkg undiluted and as a 10 aqueous solution 02 gkg diethylene glycol butyl ether. Specific GravityDensity1680 Molecular FormulaH3O4P Molecular Weight98 Section 10 - Stability and Reactivity Chemical Stability. 2-ethylhexan-1-ol is a primary alcohol that is hexan-1-ol substituted by an ethyl group at position 2.
 Source: youtube.com
Source: youtube.com
Its important to remember that common names are inaccurate and vary from one place and time to another. 730 Torr at 20C. Even so you rarely ask someone to pass the sodium chloride at the dinner table. A ternary mixture of ethyl acetate water and ethyl alcohol distils first. Some adhesives like commercial adhesives nail glue and sticker residue are tougher to remove than others because of the composition of the adhesives.
Source: quora.com
Its formula can be also written as CH 3 CH 2 OH or C 2 H 5 OH an ethyl group linked to a hydroxyl group and is often abbreviated as EtOHEthanol is a volatile flammable colorless liquid with a. Even so you rarely ask someone to pass the sodium chloride at the dinner table. Its important to remember that common names are inaccurate and vary from one place and time to another. 100 deg C FreezingMelting Point0 deg C Decomposition TemperatureNot available. 77 C Partition coefficient n- octanolwater.
 Source: researchgate.net
Source: researchgate.net
Limitsstandards shown for guidance only. 1 Structures Expand this section. Safety Data Sheet according to 29CFR19101200 and. 141-78-6 ethyl acetate PEL 400 ppm 1400 mgm3 US. Upper flammable limit19 by volume.
 Source: manualzz.com
Source: manualzz.com
Page 6 of 15 MSDS. As a result of these forces a lattice of water. Safety Data Sheet according to 29CFR19101200 and. Surface tension 240 mNm at 200 C 680 F Boiling pointBoiling range. This occurs at 212 degrees Fahrenheit or 100 degrees Celsius.
Source: chemspider.com
Upper flammable limit19 by volume. Even so you rarely ask someone to pass the sodium chloride at the dinner table. As a result of these forces a lattice of water. Chemical or scientific names are used to give an accurate description of a substances composition. The condensation point of water is the same as the boiling point of water.
Source: quora.com
Absorbs moisture or water from the air. If a fluid consist of more than one component a solution components with. Absorption and elimination of dermally applied doses of 14C-diethylene glycol butyl ether and 14C-diethylene glycol butyl ether acetate derivative were determined in Sprague-Dawley ratsThe materials were applied under occlusion for 24 hr at dose levels of 02 and 20 gkg undiluted and as a 10 aqueous solution 02 gkg diethylene glycol butyl ether. 141-78-6 ethyl acetate PEL 400 ppm 1400 mgm3 US. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that escapes from the liquid to form a separate vapor phase above the liquid surface.
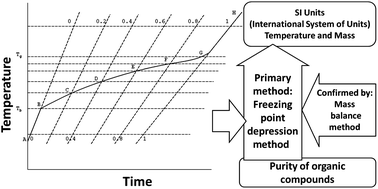
Flash point between 140 - 175F. If a fluid consist of more than one component a solution components with. 08945 gmL 7465 lbgal at 25C. Flash point between 140 - 175F. Freezing-point depression is a drop in the temperature at which a substance freezes caused when a smaller amount of another non-volatile substance is added.
 Source: researchgate.net
Source: researchgate.net
Surfactants and 2 freezing point depressants antifreezes. A ternary mixture of ethyl acetate water and ethyl alcohol distils first. Page 6 of 15 MSDS. Ethanol also called ethyl alcohol grain alcohol drinking alcohol or simply alcohol is an organic chemical compoundIt is a simple alcohol with the chemical formula C 2 H 6 O. Surfactantssuch as amines diamines amides or glycol esters of fatty acidshelp keep the water droplets dispersed by limiting coagulation.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title ethyl acetate freezing point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.