Ethyl acetate boiling point
Home » datasheet » Ethyl acetate boiling pointEthyl acetate boiling point
Ethyl Acetate Boiling Point. Boiling point of water. ETHYL ACETATE is also sensitive to heat. This is actually not the case provided that you do things the right way. Unsuitable Extinguishing MediaWater may be ineffective Do not use a solid water stream as it may scatter and spread fire Flash Point-4 C 248 F Method - Closed cup.
 Ethyl Acetate Anhydrous 99 8 141 78 6 From sigmaaldrich.com
Ethyl Acetate Anhydrous 99 8 141 78 6 From sigmaaldrich.com
Ethyl lactate also known as lactic acid ethyl ester is the organic compound with the formula CH 3 CH OHCO 2 CH 2 CH 3. The ICSC project is a common undertaking between the World Health Organization WHO and. 7837 C 1731 F Boiling point of methanol. Lower and upper explosion limit flammability limit. Read on for more information. 100 C 212 F Boiling point of water in Kelvin.
730 Torr at 20C.
56 C 1328 F Boiling point of alcohol. Ethyl acetate Revision Date 18-January-2018 5. D-Ethyl lactate is obtained from d-lactic acid by azeotropic distillation with ethyl alcohol or benzene in the presence of concentrated H2SO4. 08945 gmL 7465 lbgal at 25C. Therefore ethyl acetate will be the first fraction collected as the distillate. The general method of ester preparation can be summarised as the reversible reaction.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
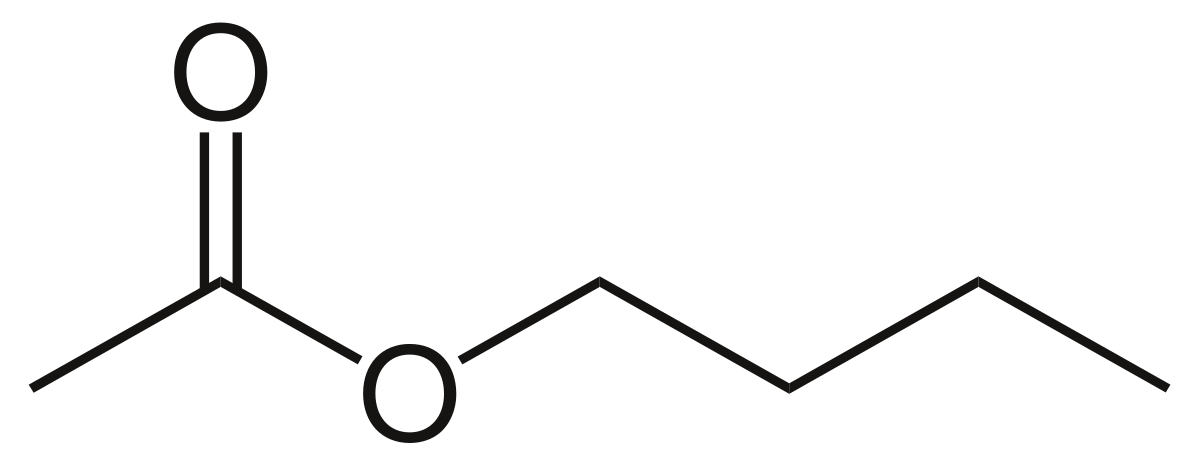
-269 C -452 F. 7837 C 1731 F Boiling point of nitrogen. A liquid boils when its vapour pressure is equal to the atmospheric pressure. Ethyl acetate systematically ethyl ethanoate commonly abbreviated EtOAc ETAC or EA is the organic compound with the formula CH 3 COOCH 2 CH 3 simplified to C 4 H 8 O 2This colorless liquid has a characteristic sweet smell similar to pear drops and is used in glues nail polish removers and in the decaffeination process of tea and coffee. Many people believe these solvents are difficult or slow to remove.
 Source: tcichemicals.com
Source: tcichemicals.com
The procedure for the distillation of the mixture containing the ester is outlined below. Water butanoles propanoles aniline toluene bromoform dimethylformamide. Suppose you are going to perform distillation under reduced. Ethyl acetate Revision Date 18-January-2018 5. Ethyl acetate Methanol Acetonitrile TFA Hexane Chloroform THF DCM Diethyl ether Other commonly used HBP solvents include NMP N-Methyl-pyrrolidone DMAc Di-Methyl-Acetamide.
Source: chemspider.com
ETHYL ACETATE is also sensitive to heat. 730 Torr at 20C. Unsuitable Extinguishing MediaWater may be ineffective Do not use a solid water stream as it may scatter and spread fire Flash Point-4 C 248 F Method - Closed cup. 09006 gmL 7516 lbgal at 20C. 001 - dihydromyrcenol 005 - benzyl propionate 005 - linalyl acetate 005 - phenethyl alcohol 001 - aldehyde C-14 10 020 - amyl cinnamaldehyde 001 - mahy shiff 005 - naphthyl ethyl ether 001 - aldehyde C-16 10 005 - geraniol an antimicrobial antiseptic and disinfectant that is used also as an aromatic essence and preservative in pharmaceutics and perfumery.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Therefore ethyl phenylacetate may volatilize from dry soil. Suppose you are going to perform distillation under reduced. -1958 C -3204 F Boiling point of liquid helium. Ethyl acetate is the ester of ethanol. Genevac Limited The Sovereign Centre.
 Source: researchgate.net
Source: researchgate.net
3732 K Boiling point of ethanol. By weight in mixture. CH3COOH C2H5OH h CH3COOC2H5 H2O. Methyl ethyl ketone. Reaction is given below.
 Source: acs.org
Source: acs.org
This chemical may ignite or explode with lithium aluminum hydride. The l-form is prepared in similar fashion starting from l-lactic acid. Should be stored in approved flammable. Its boiling point is 771. Ethyl acetate is the ester of ethanol.
Source: quora.com
A liquid boils when its vapour pressure is equal to the atmospheric pressure. 001 - dihydromyrcenol 005 - benzyl propionate 005 - linalyl acetate 005 - phenethyl alcohol 001 - aldehyde C-14 10 020 - amyl cinnamaldehyde 001 - mahy shiff 005 - naphthyl ethyl ether 001 - aldehyde C-16 10 005 - geraniol an antimicrobial antiseptic and disinfectant that is used also as an aromatic essence and preservative in pharmaceutics and perfumery. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. Therefore ethyl acetate will be the first fraction collected as the distillate. This chemical may ignite or explode with lithium aluminum hydride.
Source: chem.nlm.nih.gov
The main target users are workers and those responsible for occupational safety and health. Ethyl acetate is the ester of ethanol. Ethyl phenylacetate has an estimated vapor pressure of 624X10-2 mm Hg and exists as a liquid under environmental conditions. Boiling point helps identify and characterise a compound. 647 C 1485 F Boiling point of acetone.
 Source: researchgate.net
Source: researchgate.net
3732 K Boiling point of ethanol. It is commonly used as a. Ethyl acetate Methanol Acetonitrile TFA Hexane Chloroform THF DCM Diethyl ether Other commonly used HBP solvents include NMP N-Methyl-pyrrolidone DMAc Di-Methyl-Acetamide. Being naturally derived it is readily available as a single enantiomer. It is incompatible with nitrates strong alkalis and strong acids.
08945 gmL 7465 lbgal at 25C. The l-form is prepared in similar fashion starting from l-lactic acid. 188 D at 25C. By weight in mixture. It is commonly used as a.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title ethyl acetate boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.