Ethane boiling point
Home » datasheet » Ethane boiling pointEthane boiling point
Ethane Boiling Point. Its all about the Van der Waals interactions. The curve between the critical point and the triple point shows the ethane boiling point with changes in pressure. Explosions of such mixtures have been frequent in coal mines and collieries and have been the cause of many mine disasters. In the ABSENCE of other intermolecular force the higher the molecular mass the greater the boiling point.
 Ethane Data Page Wikipedia From en.wikipedia.org
Ethane Data Page Wikipedia From en.wikipedia.org
So isobutane is a slightly better choice in cold weather but propane is the best at -42C -44F. The curve between the critical point and the triple point shows the ethane boiling point with changes in pressure. Boiling point is related to the forces between molecules which in the case of hydrocarbons is Van Der Waals interactions. A little confused on this isnt boiling point a. That is a pretty wild fact isnt it. It is mainly used for two purposes as a raw material in the manufacture of polyester fibers and for antifreeze formulations.
So isobutane is a slightly better choice in cold weather but propane is the best at -42C -44F.
Ethane ˈ ɛ θ eɪ n or ˈ iː θ eɪ n is an organic chemical compound with chemical formula C 2 H 6At standard temperature and pressure ethane is a colorless odourless gasLike many hydrocarbons ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refiningIts chief use is as feedstock for ethylene production. If youve ever seen microscope images of a geckos feet which allow it to climb walls youll see that there is no adhesive but the pads contain a tremendous amount of surface area. Boiling point is related to the forces between molecules which in the case of hydrocarbons is Van Der Waals interactions. That is a pretty wild fact isnt it. 3 Chemical and Physical Properties Expand this section. Some fuels and their boiling points at atmospheric pressure.
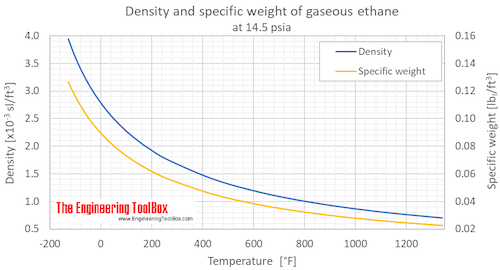 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
It is mainly used for two purposes as a raw material in the manufacture of polyester fibers and for antifreeze formulations. The boiling point of n butane is -04C 313F vs the boiling point of isobutane at -1175C 1085F. Ethylene glycol is produced from ethylene ethene via the intermediate. The metabolism of ethane to ethanol does not occur to any significant extent in rat liver microsomal preparations perhaps because ethane is a poor substrate for the cytochrome P450 enzyme system. What about the higher boiling point of methoxy ethane when compared to n-butane.
 Source: youtube.com
Source: youtube.com
2 Names and Identifiers Expand this section. CH 3 CH 2 I 2H C 2 H 6 HI. What about the higher boiling point of methoxy ethane when compared to n-butane. Soc 1930 52 611-622. Ethyl methanoate has some dipole-dipole interactions but cannot form a hydrogen bond.
 Source: thermopedia.com
Source: thermopedia.com
The boiling point of n butane is -04C 313F vs the boiling point of isobutane at -1175C 1085F. For propanoic acid hydrogen bonds form between the carbonyl group on one acid. 3 Chemical and Physical Properties Expand this section. The ethane phase diagram shows the phase behavior with changes in temperature and pressure. 1 Structures Expand this section.
Source: quora.com
2 Names and Identifiers Expand this section. The flash point is the lowest temperature at which the gas will ignite with an ignition source not to be confused with the autoignition temperature spontaneous ignition. So isobutane is a slightly better choice in cold weather but propane is the best at -42C -44F. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The heat capacity of saturated liquid ethane from the boiling point to the critical temperature and heat fusion of the solid J.
 Source: en.wikipedia.org
Source: en.wikipedia.org
When methyl bromide or methyl iodide and sodium are heated in the presence of dry ether ethane is formed. The remaining examples in the table conform to the correlation of boiling point with total electrons and number of nuclei. 2 Names and Identifiers Expand this section. Explosions of such mixtures have been frequent in coal mines and collieries and have been the cause of many mine disasters. Ethylene glycol IUPAC name.
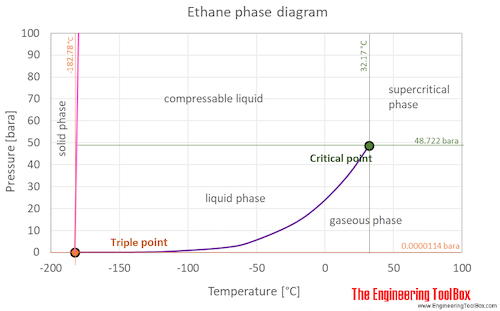 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Some fuels and their boiling points at atmospheric pressure. If youve ever seen microscope images of a geckos feet which allow it to climb walls youll see that there is no adhesive but the pads contain a tremendous amount of surface area. Refined natural gas is primarily made of methane and is commonly used for cooking. The flash point is the lowest temperature at which the gas will ignite with an ignition source not to be confused with the autoignition temperature spontaneous ignition. You would not expect that diethyl ether would have a lower boiling point than pentane.
The ethane phase diagram shows the phase behavior with changes in temperature and pressure. The boiling point of a substance is the temperature at which it can change state from a liquid to a gas throughout the bulk of the liquid. You would not expect that diethyl ether would have a lower boiling point than pentane. The flash point is the lowest temperature at which the gas will ignite with an ignition source not to be confused with the autoignition temperature spontaneous ignition. Lipid perioxidation processes can however generate ethane as an end product of degradation.
 Source: alevelchem.com
Source: alevelchem.com
What about the higher boiling point of methoxy ethane when compared to n-butane. The boiling point of a substance is the temperature at which it can change state from a liquid to a gas throughout the bulk of the liquid. The flash point is the lowest temperature at which the gas will ignite with an ignition source not to be confused with the autoignition temperature spontaneous ignition. The curve between the critical point and the triple point shows the ethane boiling point with changes in pressure. Ethane ˈ ɛ θ eɪ n or ˈ iː θ eɪ n is an organic chemical compound with chemical formula C 2 H 6At standard temperature and pressure ethane is a colorless odourless gasLike many hydrocarbons ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refiningIts chief use is as feedstock for ethylene production.
 Source: youtube.com
Source: youtube.com
The alcohol butan-1-ol can form hydrogen bonds and so has a higher boiling point. The curve between the critical point and the triple point shows the ethane boiling point with changes in pressure. A little confused on this isnt boiling point a. Ethane is a gas at standard conditions. The heat capacity of saturated liquid ethane from the boiling point to the critical temperature and heat fusion of the solid J.
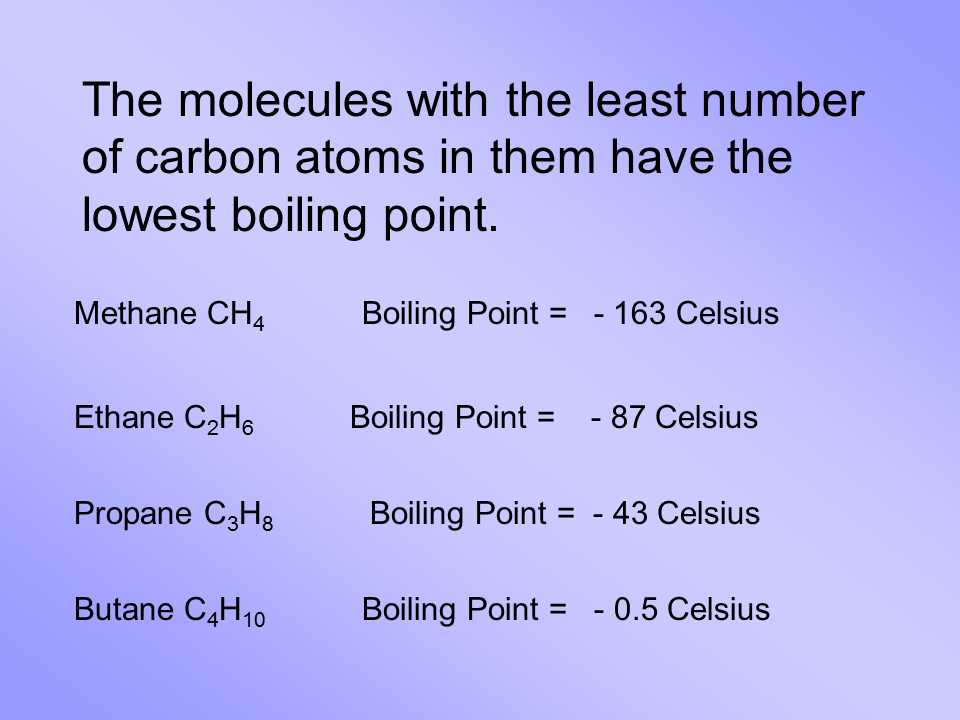
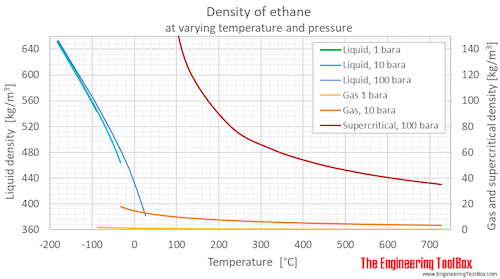 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Methane ethane propane butane etc. It is mainly used for two purposes as a raw material in the manufacture of polyester fibers and for antifreeze formulations. CH 3 CH 2 I 2H C 2 H 6 HI. What about the higher boiling point of methoxy ethane when compared to n-butane. Look at the alkane series.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title ethane boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.