Ethanal boiling point
Home » datasheet » Ethanal boiling pointEthanal boiling point
Ethanal Boiling Point. Using calorimetry to find out which type of nut butter contains most calories. Consuming large quantities of ethanol causes acetaldehyde to build up in bloodstream faster than it can be consumed in the liver to make other products leading to nausea sweating reduced blood pressure etc. Depending on the particular carbonyl species the pKa of the a-hydrogen typically falls in the range of 17. Homologous series functional group prefix suffix usual use example Alkane-ane CH3CH2CH2CH3 butane Alkenes Suffix -ene Alcohols suffix -ol prefix hydroxy-Halogenoalkanes Prefix chloro-bromo-iodo-Aldehydes suffix -al prefix formyl.
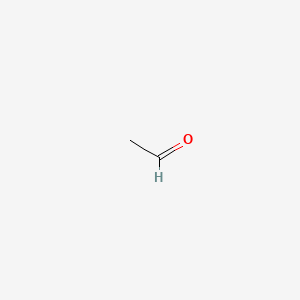
That means that ethanal boils at close to room temperature. We can see from the table that whereas the relative mass is the same between alkanes and aldehydes the boiling point of the aldehydes is much higher. Methanal is a gas boiling point -21C and ethanal has a boiling point of 21C. CH 3 CH 2 CH 2 OH. Academiaedu is a platform for academics to share research papers. The boiling point of methanal is -19 o C and for ethanal it is 21 o C.
2933 K Solubility in water.
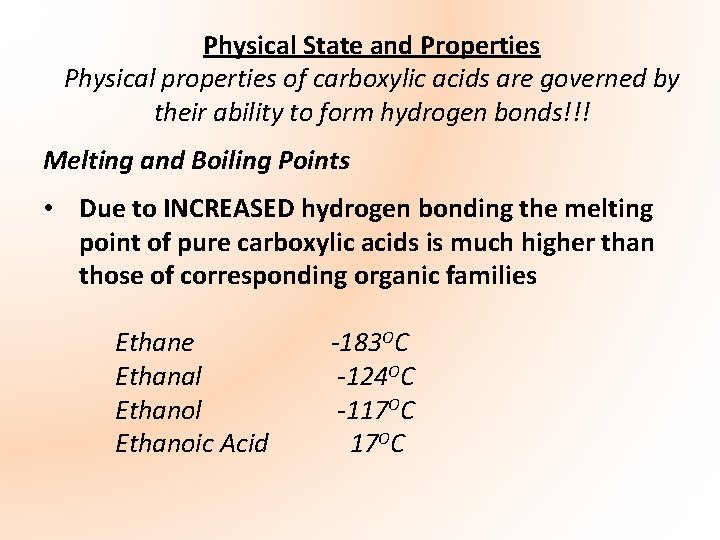
D the pH change occurs outside the range of any indicator. The other aldehydes and the ketones are liquids with boiling points rising as the molecules get bigger. Branching increases the strength of vander-waals forces. Comparing the enthalpies of combustion of ethanol ethanal and ethanoic acid using calorimetry to quantify intermolecular force strength. B there are too few H ions to affect the indicator. Boiling Point C CH 3 CH 2 CH 2 CH 3.

Boiling point depends upon the strength of the intermolecular forces. That means that ethanal boils at close to room temperature. Comparing the enthalpies of combustion of ethanol ethanal and ethanoic acid using calorimetry to quantify intermolecular force strength. Log P-034 Vapor pressure. Van der Waals dispersion forces.
 Source: slidetodoc.com
Source: slidetodoc.com
These attractions get. Used to make other chemicals. The size of the boiling point is governed by the strengths of the intermolecular forces. That means that ethanal boils at close to room temperature. Methanal also known as formaldehyde HCHO is a gas at room temperature boiling point -21C and ethanal also known as acetaldehyde has a boiling point of 21C.
 Source: chemguide.co.uk
Source: chemguide.co.uk
Van der Waals dispersion forces. Structure of the aldehyde. But formaldehyde HCHO is an aldehyde where carbonyl carbon is attached with two hydrogen atoms. 2933 K Solubility in water. The other aldehydes and the ketones are liquids with boiling points rising as the molecules get bigger.
 Source: ch.ic.ac.uk
Source: ch.ic.ac.uk
Therefore boiling point increase in the order Propan-1. 740 mmHg 20 C Acidity pK a 1357 25 C H 2 O Magnetic susceptibility χ-5153 6 cm 3 g Refractive index n D 13316 Viscosity. It is of course due to dipole- dipole forces acting between the relatively negative oxygen on. But formaldehyde HCHO is an aldehyde where carbonyl carbon is attached with two hydrogen atoms. D the pH change occurs outside the range of any indicator.
 Source: slideplayer.com
Source: slideplayer.com
The boiling point of methanal is -19 o C and for ethanal it is 21 o C. Larger aldehydes and the ketones are liquids with boiling points rising as the molecules get bigger. Methanal also known as formaldehyde HCHO is a gas at room temperature boiling point -21C and ethanal also known as acetaldehyde has a boiling point of 21C. Ethanal Acetaldehyde Product of oxidation of ethanol in the liver. So the boiling point of pentan-1-ol is more than that of all other given compounds.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The other aldehydes and the ketones are liquids with boiling points rising as the molecules get bigger. In each case reduction essentially involves the addition of a hydrogen atom to each end of the carbon-oxygen double bond to form an alcohol. Cambridge International AS and A Level Chemistry Coursebook 2nd Edition. CBSE Class 12 Chemistry Alcohols Phenols and Ethers MCQs Set B with answers available in Pdf for free download. Reduction of aldehydes and ketones lead to two different sorts of alcohol.
 Source: slideplayer.com
Source: slideplayer.com
C there are too few OH ions to affect the indicator. We can see from the table that whereas the relative mass is the same between alkanes and aldehydes the boiling point of the aldehydes is much higher. Have only single bonds. Boiling point depends upon the strength of the intermolecular forces. Academiaedu is a platform for academics to share research papers.
 Source: slideplayer.com
Source: slideplayer.com
C there are too few OH ions to affect the indicator. So the boiling point of pentan-1-ol is more than that of all other given compounds. Students of class 12 Chemistry should refer to MCQ Questions Class 12 Chemistry Alcohols Phenols and Ethers with answers provided here which is an important chapter in Class 12 Chemistry NCERT textbook. Ethanal Acetaldehyde Product of oxidation of ethanol in the liver. It has a role as a human metabolite an EC 3514 amidase inhibitor a.
Source: quora.com
Therefore boiling point increase in the order Propan-1. Same chemical properties. Ethanal Acetaldehyde Product of oxidation of ethanol in the liver. Low boiling point high flammability high melting point. The C-Cl bond in chlorobenzene shows partial double bond character due to resonance.
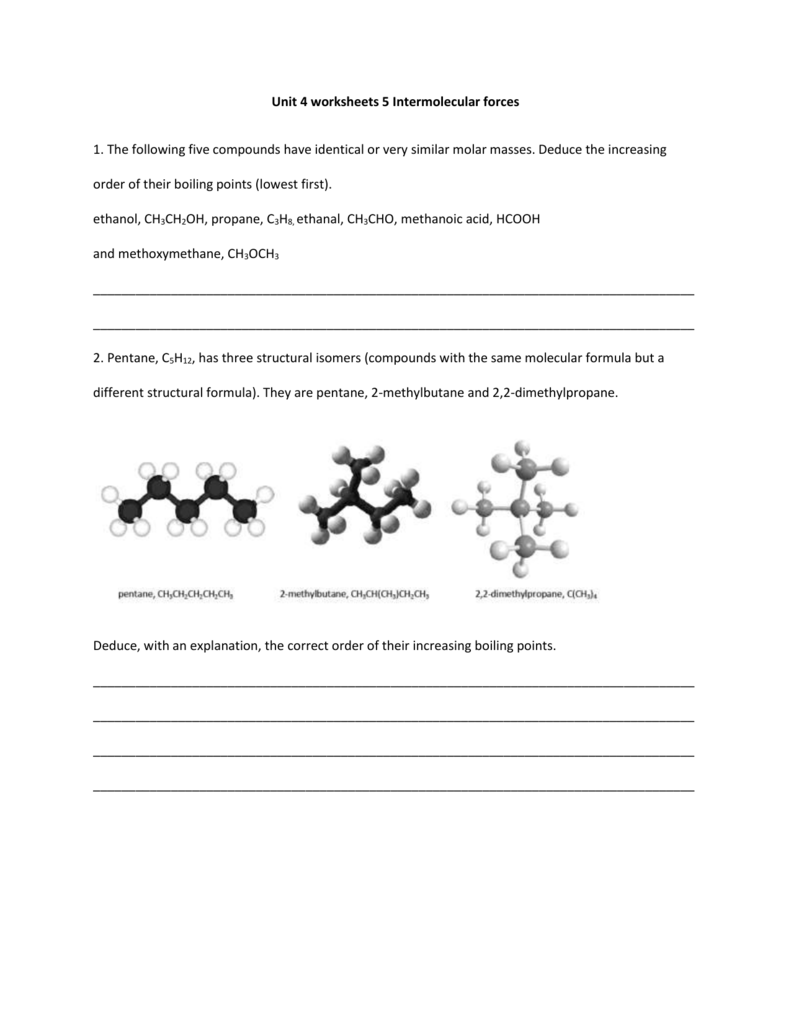 Source: studylib.net
Source: studylib.net
Consuming large quantities of ethanol causes acetaldehyde to build up in bloodstream faster than it can be consumed in the liver to make other products leading to nausea sweating reduced blood pressure etc. Used to make other chemicals. The other aldehydes and the ketones are liquids with boiling points rising as the molecules get bigger. From this we can say that the boiling point of ethanal is close to room temperature. Ethanol also called ethyl alcohol grain alcohol drinking alcohol or simply alcohol is an organic chemical compoundIt is a simple alcohol with the chemical formula C 2 H 6 O.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title ethanal boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.