Dmf boiling point
Home » datasheet » Dmf boiling pointDmf boiling point
Dmf Boiling Point. Evaporation DMF aniline toluene CALIBRATION. This time we are going to collect a particular fraction in the flask - there is DCM BP 40C water BP 100C DMF 153C safrole 232C ketone BP. To gain access to the Darkmoon Faire quests you must have at least 1 skill point in your profession. A solution of sodium ethoxide is prepared from 60 g.
 Dmso Physical Properties Gaylord Chemical From gaylordchemical.com
Dmso Physical Properties Gaylord Chemical From gaylordchemical.com
D7398 - 112021 Standard Test Method for Boiling Range. Small amounts of low-boiling-point solvents like diethyl ether dichloromethane or acetone will evaporate in seconds at room temperature while high-boiling-point solvents like water or dimethyl sulfoxide need higher temperatures an air flow or the application of vacuum for fast evaporation. Well this could be happening several different ways but one pathway is reductive. The table above distinguishes between protic and aprotic solvents. Pour the DCMOrganic layer into your CLEAN 500mL RBFlask. Pressure unit must be identical for both values.
The boiling point is an important property because it determines the speed of evaporation.
H 3 L3 30 mg 0048 mmol and 10 drops of 12-ethanedithiol 170 mg 181 mmol were dissolved in DMF 30 ml while ZrOCl 2 8H 2 O 92 mg 029 mmol and formic acid. Cite 25th Oct 2017. It is a colourless liquid with a high boiling point which is miscible with water and also with a majority of common organic solvents. The boiling point is an important property because it determines the speed of evaporation. The products are then dissolved in water and the pH is adjusted to above 12 with the addition of NaOH in order to neutralize the hydrohalide salt. Thionyl chloride is a well-suited reagent as the by-products HCl SO 2 are gases and residual thionyl chloride can be easily removed as a result of its low boiling point 76 CThe reaction with thionyl chloride is catalyzed by dimethylformamide.
 Source: researchgate.net
Source: researchgate.net
H 3 L3 30 mg 0048 mmol and 10 drops of 12-ethanedithiol 170 mg 181 mmol were dissolved in DMF 30 ml while ZrOCl 2 8H 2 O 92 mg 029 mmol and formic acid. D7213 - 152019 Standard Test Method for Boiling Range Distribution of Petroleum Distillates in the Boiling Range from 100 C to 615 C by Gas Chromatography. A solution of sodium ethoxide is prepared from 60 g. To gain access to the Darkmoon Faire quests you must have at least 1 skill point in your profession. H 3 L3 30 mg 0048 mmol and 10 drops of 12-ethanedithiol 170 mg 181 mmol were dissolved in DMF 30 ml while ZrOCl 2 8H 2 O 92 mg 029 mmol and formic acid.
 Source: americanlaboratory.com
Source: americanlaboratory.com
Put several boiling stones in too. 10057 gml 20C Refractive Index. The products can be isolated either by precipitation with ethyl ether or by evaporation of the methanol solvent. Ether hexanes carbon disulfide methylene chloride. Thionyl chloride is a well-suited reagent as the by-products HCl SO 2 are gases and residual thionyl chloride can be easily removed as a result of its low boiling point 76 CThe reaction with thionyl chloride is catalyzed by dimethylformamide.
 Source: biochromato.com
Source: biochromato.com
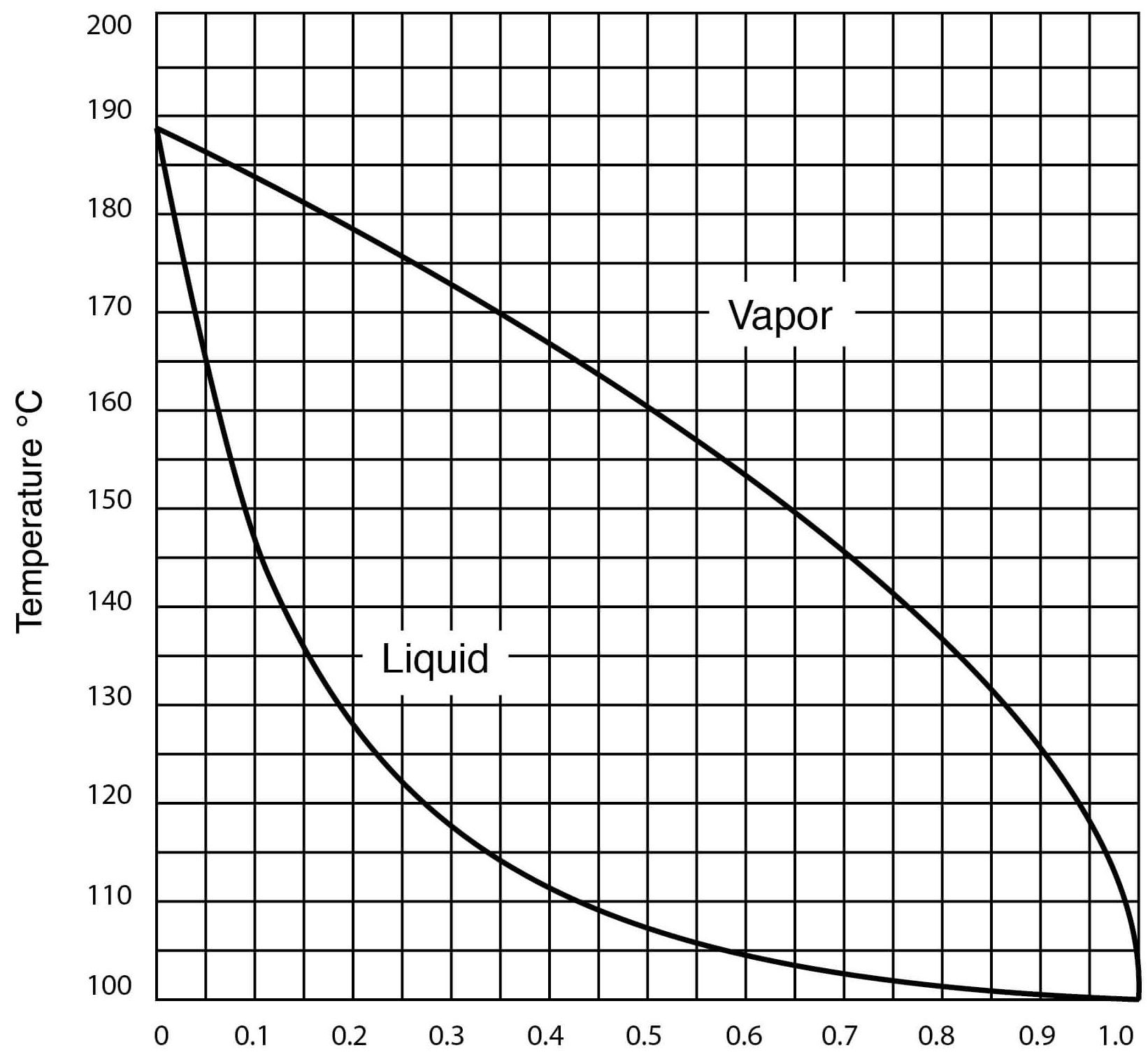
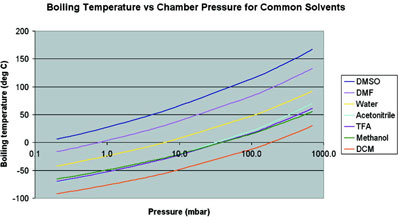
Ill try to keep it as updated once I have. Cite 25th Oct 2017. 10057 gml 20C Refractive Index. DFM is a blend of diesel fuel that is basically the same as kerosene to which high-boiling-point fractions and high-boiling-point residual oils have been added. The graph below shows a range of solvents with two well known HBP solvents DMF Dimethylformamide DMSO Dimethylsulfoxide occurring to the left of water which is in yellow on the graph.
 Source: researchgate.net
Source: researchgate.net
Ill try to keep it as updated once I have. Thionyl chloride is a well-suited reagent as the by-products HCl SO 2 are gases and residual thionyl chloride can be easily removed as a result of its low boiling point 76 CThe reaction with thionyl chloride is catalyzed by dimethylformamide. 10057 gml 20C Refractive Index. D7398 - 112021 Standard Test Method for Boiling Range. March 23 2015 at 845 am.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The rewarded 5 skill points are distributed based on the newest expansion you have training in that is not currently maxed out. Boiling Temperature vs Chamber Pressure for Common Solvents-40-30-20-10 0 10 20 30 40 50 60 010 100 1000 10000. Above enter the boiling point under the other pressure. Ill try to keep it as updated once I have. JP-8 was developed for the Air Force to provide a safe kerosene-based jet fuel that would still have adequate reliability and an acceptable freezing point.
 Source: americanlaboratory.com
Source: americanlaboratory.com
It has a role as a fossil fuel a member of greenhouse gas and a bacterial metabolite. For the solvents included in the table the distinguishing feature is the presence of an -OH group and that is the most common characteristic of a protic solvent. DFM is a blend of diesel fuel that is basically the same as kerosene to which high-boiling-point fractions and high-boiling-point residual oils have been added. Evaporation DMF aniline toluene CALIBRATION. At this point the product will be a mixture of the desired tertiary amine and the undesired quaternary ammonium compound.
 Source: gaylordchemical.com
Source: gaylordchemical.com
Methane is a one-carbon compound in which the carbon is attached by single bonds to four hydrogen atomsIt is a colourless odourless non-toxic but flammable gas bp. The products are then dissolved in water and the pH is adjusted to above 12 with the addition of NaOH in order to neutralize the hydrohalide salt. Of course boiling point relationships may be dominated by even stronger attractive forces such as those involving electrostatic attraction between oppositely charged ionic species and between the partial charge separations of molecular dipoles. Pressure unit must be identical for both values. 26 mol clean sodium and 700 ml of absolute alcohol dried over calcium oxide or sodium in a 2000 ml round.
 Source: biopharma.co.uk
Source: biopharma.co.uk
The table above distinguishes between protic and aprotic solvents. Introduction N N-Dimethylformamide DMF is an extraordinary organic compound with the formula CH 3 2 NCOH. Methane is a one-carbon compound in which the carbon is attached by single bonds to four hydrogen atomsIt is a colourless odourless non-toxic but flammable gas bp. A solution of sodium ethoxide is prepared from 60 g. D7213 - 152019 Standard Test Method for Boiling Range Distribution of Petroleum Distillates in the Boiling Range from 100 C to 615 C by Gas Chromatography.
 Source: researchgate.net
Source: researchgate.net
Pressure unit must be identical for both values. The rewarded 5 skill points are distributed based on the newest expansion you have training in that is not currently maxed out. A solution of sodium ethoxide is prepared from 60 g. The graph below shows a range of solvents with two well known HBP solvents DMF Dimethylformamide DMSO Dimethylsulfoxide occurring to the left of water which is in yellow on the graph. The products are then dissolved in water and the pH is adjusted to above 12 with the addition of NaOH in order to neutralize the hydrohalide salt.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Nice high boiling point. Boiling Temperature vs Chamber Pressure for Common Solvents-40-30-20-10 0 10 20 30 40 50 60 010 100 1000 10000. Set up for vacuum distillation. Carbon tet is great if you can find it. In addition to the above you can use special boiling point SBP solvent which consists of C6 hydrocarbons and is a byproduct of NGL recovery plants.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title dmf boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.