Dimethylpropane boiling point
Home » datasheet » Dimethylpropane boiling pointDimethylpropane boiling point
Dimethylpropane Boiling Point. For example 22-dimethylpropane neopentane has a low boiling point than pentane. The surrounding temperature around a storage tank should always. CRYSTALS OR COLOURLESS LIQUID. The intermolecular forces are both London forces and permanent dipole-dipole attractions.
 Chemistry Organic Molecules Trends In Boiling Temp Viscosity And Flash Point From dynamicscience.com.au
Chemistry Organic Molecules Trends In Boiling Temp Viscosity And Flash Point From dynamicscience.com.au
Generally flash point increases with an increase in boiling point. The intermolecular forces are both London forces and permanent dipole-dipole attractions. ILO International Chemical Safety Cards ICSC A clear light colored liquid. The boiling point of a liquid varies depending upon the surrounding environmental pressure. 2-methylbutane 22-dimethylpropane. One side of the molecule is slightly negative.
Flash point is an important parameter for safety considerations especially during storage and transportation of volatile petroleum products ie LPG light naphtha gasoline in a high-temperature environment.
ILO International Chemical Safety Cards ICSC A clear light colored liquid. The surrounding temperature around a storage tank should always. Iii 1-Bromobutane 1-Bromo-2-methylbutane 1-Bromo-2 2-dimethylpropane. The intermolecular forces found in 1-butanol an alcohol are the strongest leading to the highest boiling point. 2-methylbutane 22-dimethylpropane. For example 22-dimethylpropane neopentane has a low boiling point than pentane.

Chlorine withdraws electrons through inductive effect and releases through resonance. The intermolecular forces are both London forces and permanent dipole-dipole attractions. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. For example 22-dimethylpropane neopentane has a low boiling point than pentane. Used to make other chemicals.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
EPA Chemicals under the TSCA. Explain why it is so. The polar C-Cl bonds are on the same side of the molecule. Used to make other chemicals. For example 22-dimethylpropane neopentane has a low boiling point than pentane.
 Source: chemsynthesis.com
Source: chemsynthesis.com
The intermolecular forces found in 1-butanol an alcohol are the strongest leading to the highest boiling point. The surrounding temperature around a storage tank should always. The polar C-Cl bonds are on opposite. One side of the molecule is slightly negative. CRYSTALS OR COLOURLESS LIQUID.

One side of the molecule is slightly negative. Hence it occupies less surface area than the more cylindrical pentane molecule. The boiling point of a liquid varies depending upon the surrounding environmental pressure. Although chlorine is an electron withdrawing group yet it is ortho- para-directing in electrophilic aromatic substitution reactions. Use of the information documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice and subject to other binding limitations provided for under applicable law the information documents and data made available on the ECHA website may be reproduced distributed andor used totally or in part for non-commercial purposes provided that ECHA is.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
The polar C-Cl bonds are on opposite. Explain why it is so. The polar C-Cl bonds are on opposite. ILO International Chemical Safety Cards ICSC A clear light colored liquid. The intermolecular forces are both London forces and permanent dipole-dipole attractions.

A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. Explain why it is so. Linked to first mark point accept it is an alkane or it is a saturated hydrocarbon accept converse statement 1 ii ethene 4 x single C-H bonds 1 x This is an open access article published under an ACS AuthorChoice License which permits copying and redistribution of the. The intermolecular forces found in 1-butanol an alcohol are the strongest leading to the highest boiling point. Flash point is an important parameter for safety considerations especially during storage and transportation of volatile petroleum products ie LPG light naphtha gasoline in a high-temperature environment.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Although chlorine is an electron withdrawing group yet it is ortho- para-directing in electrophilic aromatic substitution reactions. EPA Chemicals under the TSCA. The polar C-Cl bonds are on opposite. Hence it occupies less surface area than the more cylindrical pentane molecule. Merck Co Inc 1976.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
The intermolecular forces found in 1-butanol an alcohol are the strongest leading to the highest boiling point. Used to make other chemicals. Boiling point 60oC This molecule is polar. The polar C-Cl bonds are on opposite. ILO International Chemical Safety Cards ICSC A clear light colored liquid.
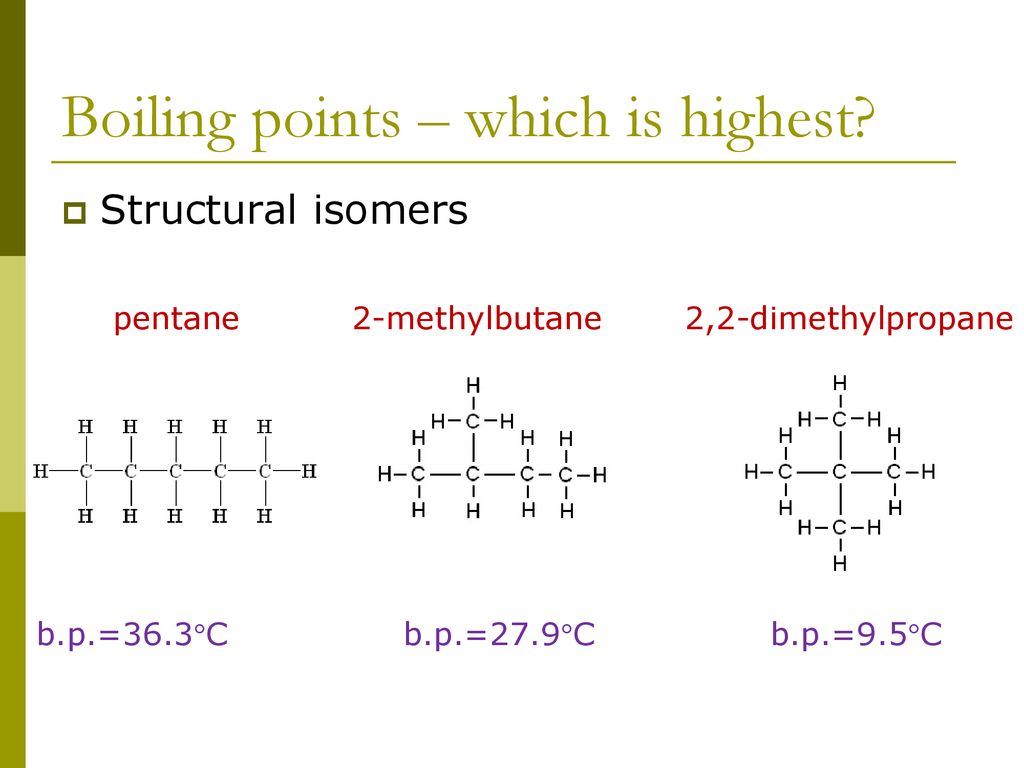 Source: slideplayer.com
Source: slideplayer.com
One side of the molecule is slightly negative. Although Cl shows -1 effect but through. Use of the information documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice and subject to other binding limitations provided for under applicable law the information documents and data made available on the ECHA website may be reproduced distributed andor used totally or in part for non-commercial purposes provided that ECHA is. Hence it occupies less surface area than the more cylindrical pentane molecule. As a result the van der Waals forces are smaller in neopentane and the boiling point is low.
 Source: chemsynthesis.com
Source: chemsynthesis.com
The intermolecular forces found in 1-butanol an alcohol are the strongest leading to the highest boiling point. The boiling point of a liquid varies depending upon the surrounding environmental pressure. Generally the boiling points of isomeric alkanes depend on their shapes. Explain why it is so. Linked to first mark point accept it is an alkane or it is a saturated hydrocarbon accept converse statement 1 ii ethene 4 x single C-H bonds 1 x This is an open access article published under an ACS AuthorChoice License which permits copying and redistribution of the.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title dimethylpropane boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.