Dimethylamine boiling point
Home » datasheet » Dimethylamine boiling pointDimethylamine boiling point
Dimethylamine Boiling Point. Secondary amines still form hydrogen bonds but having the. In the absence of a common ingredient name a term as contained in a generally accepted nomenclature shall be used. The information mentioned in point g of paragraph 1 shall be expressed by using the common ingredient name set out in the glossary provided for in Article 33. Trimethylammonium chloride is a.
 Experimental Flash Points Of Aqueous Dimethylamine Solutions As A Download Scientific Diagram From researchgate.net
Experimental Flash Points Of Aqueous Dimethylamine Solutions As A Download Scientific Diagram From researchgate.net
In the labelling making available on the market and advertising of cosmetic products. Preheat redistilled reagent-grade heptane boiling point 208 degF carefully in a clean Pyrex flask on a water bath or hot plate in a well-ventilated hood to 120 degF. Limits M-factors Notes ATP insertedATP Updated Hazard Class and Category Codes. This pressure cooker is to serve only as a container for the heptane-containing test package inside the incubator in order to minimize the danger of. Physical Thermo-dynamic Environmental. Trimethylamine TMA is an organic compound with the formula NCH 3 3It is a colorless hygroscopic and flammable tertiary amineIt is a gas at room temperature but is usually sold as a 40 solution in water.
In the labelling making available on the market and advertising of cosmetic products.
The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. It is one of the simplest members of a large class of N-nitrosaminesIt is a volatile yellow oil. For a fair comparison you would have to compare the boiling point of dimethylamine with that of ethylamine. Index No International Chemical Identification EC No CAS No Classification Labelling Specific Conc. This pressure cooker is to serve only as a container for the heptane-containing test package inside the incubator in order to minimize the danger of. The main target users are workers and those responsible for occupational safety and health.
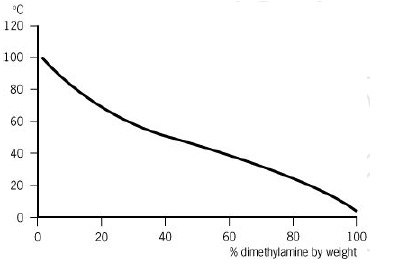 Source: eastman.com
Source: eastman.com
Then allow it to cool slowly to room temperature to crystallize and then place the flask in an ice bath to get it as cold as possible. N-Nitrosodimethylamine NDMA also known as dimethylnitrosamine DMN is an organic compound with the formula CH 3 2 NNO. Allow the crystals to form in an undisturbed flask. Attack is in the. Of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1.
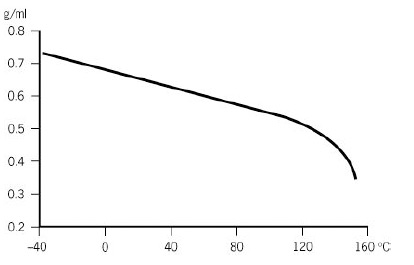 Source: eastman.com
Source: eastman.com
Yaws CL Chemical Properties Handbook. For a fair comparison you would have to compare the boiling point of dimethylamine with that of ethylamine. Trimethylamine TMA is an organic compound with the formula NCH 3 3It is a colorless hygroscopic and flammable tertiary amineIt is a gas at room temperature but is usually sold as a 40 solution in water. Allow the crystals to form in an undisturbed flask. The boiling point of the secondary amine is a little lower than the corresponding primary amine with the same number of carbon atoms.
Source: stenutz.eu
The main target users are workers and those responsible for occupational safety and health. Of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1. It is also sold in pressurized gas cylindersTMA is a nitrogenous base and can be readily protonated to give the trimethylammonium cation. How to make methylamine. It is one of the simplest members of a large class of N-nitrosaminesIt is a volatile yellow oil.
 Source: researchgate.net
Source: researchgate.net
Attack is in the. It is also sold in pressurized gas cylindersTMA is a nitrogenous base and can be readily protonated to give the trimethylammonium cation. The primary aim of the cards is to promote the safe use of chemicals in the workplace. The nitrogen content of the copolymer shall be 94 to 108 weight percent on a dry basis and a 10 percent by weight aqueous solution of the final product. How to make methylamine.
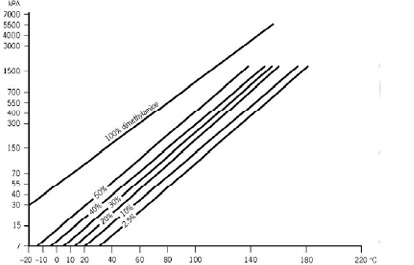 Source: eastman.com
Source: eastman.com
This pressure cooker is to serve only as a container for the heptane-containing test package inside the incubator in order to minimize the danger of. The information mentioned in point g of paragraph 1 shall be expressed by using the common ingredient name set out in the glossary provided for in Article 33. For a fair comparison you would have to compare the boiling point of dimethylamine with that of ethylamine. N-Nitrosodimethylamine NDMA also known as dimethylnitrosamine DMN is an organic compound with the formula CH 3 2 NNO. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way.
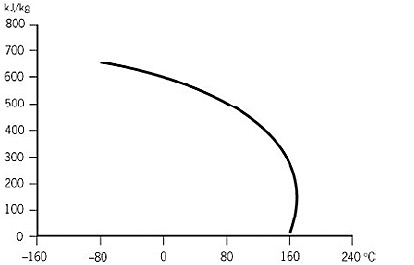 Source: eastman.com
Source: eastman.com
Attack is in the. It is also sold in pressurized gas cylindersTMA is a nitrogenous base and can be readily protonated to give the trimethylammonium cation. The ICSC project is a common undertaking between the World Health Organization WHO and. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. Material does not dissolve when the solution is heated to boiling add more water as needed.
 Source: researchgate.net
Source: researchgate.net
Then allow it to cool slowly to room temperature to crystallize and then place the flask in an ice bath to get it as cold as possible. It is one of the simplest members of a large class of N-nitrosaminesIt is a volatile yellow oil. Material does not dissolve when the solution is heated to boiling add more water as needed. The main target users are workers and those responsible for occupational safety and health. Attack is in the.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Preheat redistilled reagent-grade heptane boiling point 208 degF carefully in a clean Pyrex flask on a water bath or hot plate in a well-ventilated hood to 120 degF. Trimethylammonium chloride is a. Then allow it to cool slowly to room temperature to crystallize and then place the flask in an ice bath to get it as cold as possible. At the same time preheat a pressure cooker to 120 degF in an incubator. Limits M-factors Notes ATP insertedATP Updated Hazard Class and Category Codes.
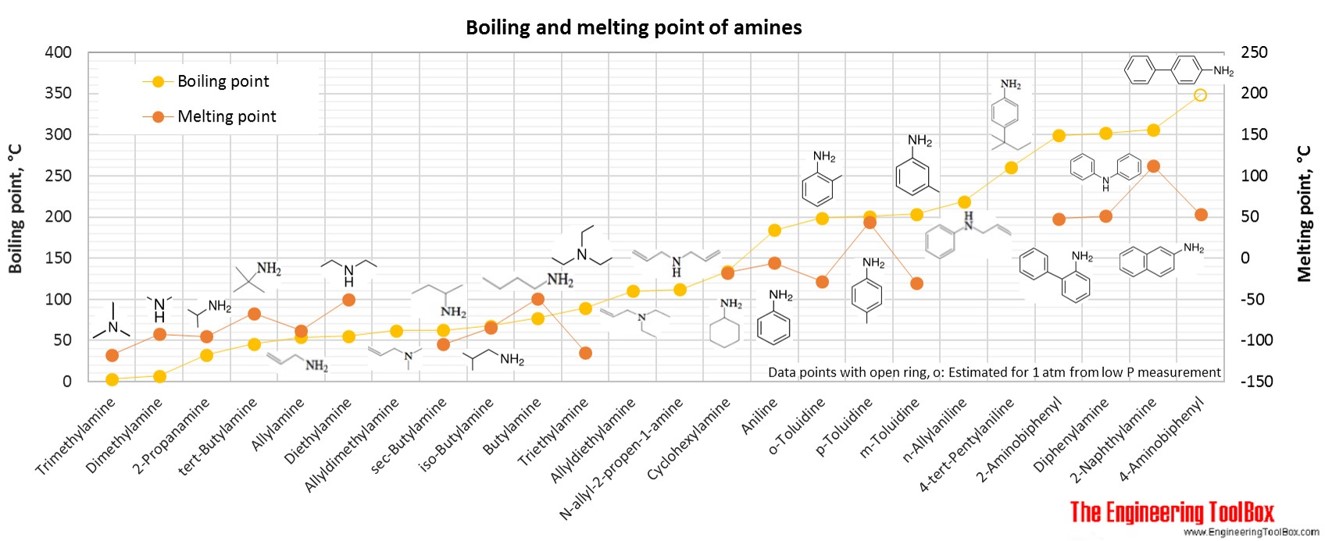 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
It is one of the simplest members of a large class of N-nitrosaminesIt is a volatile yellow oil. They are isomers of each other - each contains exactly the same number of the same atoms. This pressure cooker is to serve only as a container for the heptane-containing test package inside the incubator in order to minimize the danger of. It is one of the simplest members of a large class of N-nitrosaminesIt is a volatile yellow oil. The boiling point of the secondary amine is a little lower than the corresponding primary amine with the same number of carbon atoms.
 Source: wikidata.org
Source: wikidata.org
Physical Thermo-dynamic Environmental. It is also sold in pressurized gas cylindersTMA is a nitrogenous base and can be readily protonated to give the trimethylammonium cation. The primary aim of the cards is to promote the safe use of chemicals in the workplace. Dimethylamine-epichlorohydrin copolymer in which not more than 5 mole-percent of dimethylamine may be replaced by an equimolar amount of ethylenediamine and in which the ratio of total amine to epichlorohydrin does not exceed 11. At the same time preheat a pressure cooker to 120 degF in an incubator.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title dimethylamine boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.