Diethylene glycol boiling point
Home » datasheet » Diethylene glycol boiling pointDiethylene glycol boiling point
Diethylene Glycol Boiling Point. Public Affairs Page 1 of 5 Form Number. The urine was the major pathway for eliminating diethylene glycol monobutyl ether acetate derived 14C activity. A small amount less than 1 is used to control insects in some stored agricultural products and a very small amount is used in hospitals to sterilize medical. APPLICATIONS OF MONOETHYLENE GLYCOL Mono-ethylene Glycol MEG can be used for applications that require chemical intermediates for resins solvent couplers freezing point depression solvents humectants and chemical.

Public Affairs Page 1 of 5 Form Number. These applications are vital to the. Our MEG can be used for applications that require chemical intermediates for resins solvent couplers freezing point depression solvents humectants and chemical intermediates. Includes mineral fiber emissions from facilities manufacturing or processing. By heating the glycol in the still and reboiler to near its boiling point the glycol releases virtually all of the absorbed water and any other compounds and is then cooled for reuse. DEG is a widely used solvent.
2-2-Butoxyethoxy ethanol Diethylene glycol monobutyl ether 112-34-5 X Chemical Name CAS-No Pennsylvania 2-2-Butoxyethoxy ethanol Diethylene glycol monobutyl ether 112-34-5 X 2-Hexyloxyethanol 112-25-4 X International Inventories Page 6 7 92157794_PROF_NG-Spic and Span Revision Date.
DEG is a widely used solvent. These applications are vital to the. Benzoic acid its salts and esters are used as preservatives in cosmetic products. Glycols are key intermediates in the production of polyester fibres packaging and resins and are also used in everything from antifreezes and. DEG is a widely used solvent. Ethylene oxide is a flammable gas with a somewhat sweet odor.
 Source: hm-gt.com
Source: hm-gt.com
Industrial products made from ethylene oxide globally 17 million tonnes per annum From Devanney 2007. Yet Butyl CARBITOL glycol ether offers 100 water solubility. It dissolves easily in waterEthylene oxide is a man-made chemical that is used primarily to make ethylene glycol a chemical used to make antifreeze and polyester. 80 was eliminated after 24 hr. Hydrophobicity of the solvent package.
The fire tube is always submerged in the glycol by having the glycol flowing from the reboiler over a weir or pipe. The upper layer usually contains the. It is a colorless practically odorless poisonous and hygroscopic liquid with a sweetish taste. This has resulted in. Molecular Weight gmol 1622 Boiling point 760 mmHg 101 bar 446F 230C Flash point 210F 99C Freezing point -90F -68C.
 Source: chemicalbook.com
Source: chemicalbook.com
It is a colorless practically odorless poisonous and hygroscopic liquid with a sweetish taste. It can be a contaminant in consumer products. Our MEG can be used for applications that require chemical intermediates for resins solvent couplers freezing point depression solvents humectants and chemical intermediates. In vitro diethylene glycol monobutyl ether acetate was rapidly hydrolyzed by rat blood to diethylene glycol monobutyl ether. Molecular Weight gmol 1622 Boiling point 760 mmHg 101 bar 446F 230C Flash point 210F 99C Freezing point -90F -68C.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The heat is usually supplied through a fire tube in the reboiler in which natural gas is burned. Hydrophobicity of the solvent package. The fire tube is always submerged in the glycol by having the glycol flowing from the reboiler over a weir or pipe. It is a long term process and after the reaction one or two layers of the mixture are obtained. These applications are vital to the.
Source: chemspider.com
Hydrophobicity of the solvent package. The biological half-life was less than 3 min. And diethylene glycol when and where you need it. Diethylene glycol butyl ether 2-2-Butoxyethoxyethanol is an organic compound one of several glycol ether solvents. These applications are vital to the.
Source: merckmillipore.com
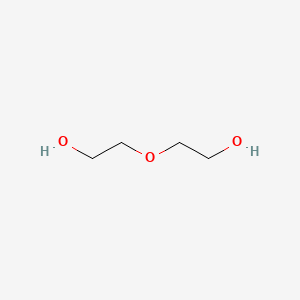
Benzoic acid its salts and esters are used as preservatives in cosmetic products. It is mainly used as a solvent for paints and varnishes in the chemical industry household detergents brewing chemicals and textile processing. 2-2-Butoxyethoxy ethanol Diethylene glycol monobutyl ether 112-34-5 X Chemical Name CAS-No Pennsylvania 2-2-Butoxyethoxy ethanol Diethylene glycol monobutyl ether 112-34-5 X 2-Hexyloxyethanol 112-25-4 X International Inventories Page 6 7 92157794_PROF_NG-Spic and Span Revision Date. Diethylene glycol DEG is an organic compound with the formula HOCH 2 CH 2 2 O. This product is reacted with a glycol normally diethylene glycol DEG ethylene glycol EG or poly ethylene glycol PEG in a reactor at high temperature.

It can be a contaminant in consumer products. APPLICATIONS OF MONOETHYLENE GLYCOL Mono-ethylene Glycol MEG can be used for applications that require chemical intermediates for resins solvent couplers freezing point depression solvents humectants and chemical. Diethylene glycol butyl ether 2-2-Butoxyethoxyethanol is an organic compound one of several glycol ether solvents. 80 was eliminated after 24 hr. The biological half-life was less than 3 min.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Diethylene glycol butyl ether 2-2-Butoxyethoxyethanol is an organic compound one of several glycol ether solvents. This product is reacted with a glycol normally diethylene glycol DEG ethylene glycol EG or poly ethylene glycol PEG in a reactor at high temperature. Includes mineral fiber emissions from facilities manufacturing or processing. 4649C Flash point. By heating the glycol in the still and reboiler to near its boiling point the glycol releases virtually all of the absorbed water and any other compounds and is then cooled for reuse.
Source: stenutz.eu
By heating the glycol in the still and reboiler to near its boiling point the glycol releases virtually all of the absorbed water and any other compounds and is then cooled for reuse. This has resulted in. These applications are vital to the. Glycol ethers include moni- and di- ethers of ethylene glycol diethylene glycol and triethylene glycol R-OCH2CH2 -OR where n 1 2 or 3 R alkyl or aryl groups R R H or groups which when removed yield glycol ethers with the structure. It can be a contaminant in consumer products.
Source: researchgate.net
Ethylene oxide is a flammable gas with a somewhat sweet odor. Hydrophobicity of the solvent package. APPLICATIONS OF MONOETHYLENE GLYCOL Mono-ethylene Glycol MEG can be used for applications that require chemical intermediates for resins solvent couplers freezing point depression solvents humectants and chemical. Here both reactant and solvent are glycol so an optimum ratio of PU and glycol should be maintained. Diethylene glycol DEG is an organic compound with the formula HOCH 2 CH 2 2 O.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title diethylene glycol boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.