Diethylamine boiling point
Home » datasheet » Diethylamine boiling pointDiethylamine boiling point
Diethylamine Boiling Point. Alcohols and carboxylic acids - physical data - Molweight melting and boiling point density pKa-values as well as number of carbon and hydrogen atoms in each. The chemicals are 100 concentrated unless stated otherwise. It is also possible to prepare it by the reaction of diethylamine and ethylene chlorohydrin. Another group of amines the biogenic amines ethanolamine histamine tyramine putrescine and cadaverine are found in many products fermented by lactic acid bacteria or in poorly conserved food.
Diethylamine Cas 109 89 7 845028 From merckmillipore.com
1179 25 August 1995. Another group of amines the biogenic amines ethanolamine histamine tyramine putrescine and cadaverine are found in many products fermented by lactic acid bacteria or in poorly conserved food. C 2 H 5 2 NH cycloCH 2 CH 2O C 2 H 5 2 NCH 2 CH 2 OH. Dissolves in 1 h. Trimethylammonium chloride is a. Table of Chemical Resistance of Flexible Graphite.
The Minister of Labour has under section 43 of the Occupational Health and Safety Act 1993 Act No.
Any glovechemical combination does not meet either set of conditions required for a GREEN or RED rating. The Minister of Labour has under section 43 of the Occupational Health and Safety Act 1993 Act No. The main target users are workers and those responsible for occupational safety and health. Part of the condensed water is cooled and reused in the quench. All of the compounds have about the same molecular weight propanoic acid diethylamine 1-butanol ethyldimethylamine 22 Examples. Alcohols and carboxylic acids - physical data - Molweight melting and boiling point density pKa-values as well as number of carbon and hydrogen atoms in each.

Let stand for 2-3 hours in the 5-10 cooling mixture. Fuels - Densities and Specific Volumes - Densities and specific volumes fuels like anthracite butane gasoil diesel coke oil wood and more. Thiocolchicoside is a semi-synthetic colchicine derivative used as an analgesic and anti-inflammatory. The temperatures are up to the boiling point of the chemical or the melting point of the metal. Lidocaine stabilizes the neuronal membrane by binding to and inhibiting voltage-gated sodium channels thereby inhibiting the ionic fluxes required for the initiation and conduction of.
Source: stenutz.eu
The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. 85 of 1993 after consultation with the Advisory Council for Occupational Health and Safety made the regulations in the Schedule. 2-aminopropane or 2-aminohexane. Table of Chemical Resistance of Flexible Graphite. The values in the table below except as noted have been extracted from online and hardbound compilations.
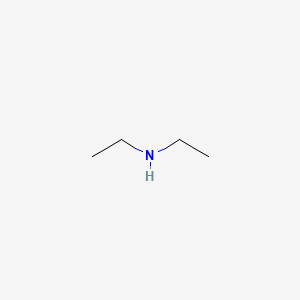
Neutralize the mixture by adding cold dilute sulfuric acid to a congo red end point pH 42. Dissolves in 1 h. The absorbance of the 50 percent alcohol when measured versus distilled water in the reference cell shall not exceed 005 at any point in the wavelength region of 245 to 310 mµ. Fuels - Boiling Points - Fuels and their boiling points. Tertiary amines have no hydrogen atom bonded to the nitrogen atom and so cannot participate in intermolecular hydrogen bonding.
 Source: wikidata.org
Source: wikidata.org
The product is first cooled to 50 C in a heat exchanger and then enters into a quench tower. Dissolves in 1 h. Free Books Science Distillation Principles And Processes List Of Known Azeotropic Mixtures. Diethylethanolamine is an irritant to the eyes skin and respiratory system. Predicting Physical Properties Which member of each of the following pairs of compounds would you expect to have a higher boiling point.
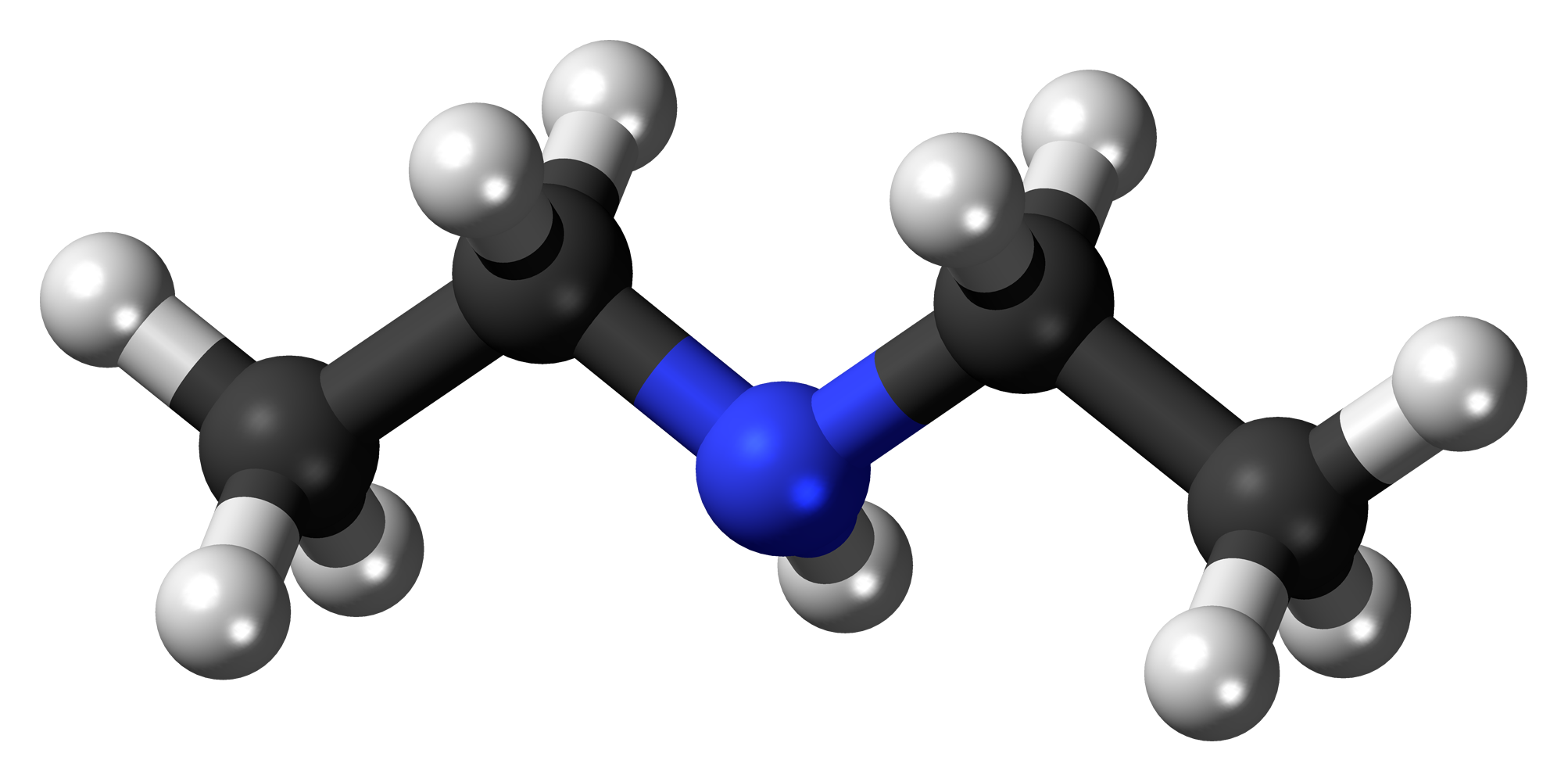 Source: en.wikipedia.org
Source: en.wikipedia.org
Based on all these data a structural suggestion for an unknown compound can be made. Break up the filter cake and put in a 2 liter beaker. Trimethylammonium chloride is a. Predicting Physical Properties Which member of each of the following pairs of compounds would you expect to have a higher boiling point. In order to determine the nature of an unknown compound correctly other techniques such as NMR 1 H 13 C HETCOR DEPT Mass spectrometry the chemical reactivity solubility tests sometimes derivatives and physical properties melting point boiling point refractive index are required as well.
 Source: en.wikipedia.org
Source: en.wikipedia.org
A glovechemical combination receives a RED rating if. Academiaedu is a platform for academics to share research papers. Hi ionic or molecular. Tertiary amines have no hydrogen atom bonded to the nitrogen atom and so cannot participate in intermolecular hydrogen bonding. Diethylethanolamine is an irritant to the eyes skin and respiratory system.
 Source: fishersci.fi
Source: fishersci.fi
Let stand for 2-3 hours in the 5-10 cooling mixture. The big difference between the boiling point of water and ethylene makes the separation process easy. 89C and methanol CH 3 OH. Academiaedu is a platform for academics to share research papers. Completely Resistant o Moderately Resistant Not Resistant x.
Source: merckmillipore.com
Lysergic acid and potassium sulphate will be seen to precipitate. The chemicals are 100 concentrated unless stated otherwise. The values in the table below except as noted have been extracted from online and hardbound compilations. Completely Resistant o Moderately Resistant Not Resistant x. This section is from the book Distillation Principles And Processes by Sydney Young.
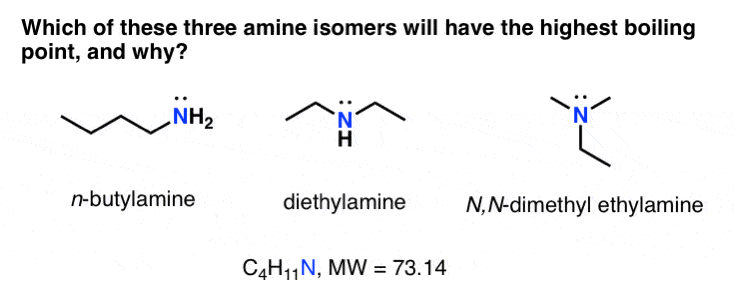 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Predicting Physical Properties Which member of each of the following pairs of compounds would you expect to have a higher boiling point. MLg Maximum extractable fraction in selected solvents expressed in percent by weight of resin Water 95 percent ethyl alcohol Ethyl acetate Benzene 1. A glovechemical combination receives a RED rating if. The big difference between the boiling point of water and ethylene makes the separation process easy. Tertiary amines have no hydrogen atom bonded to the nitrogen atom and so cannot participate in intermolecular hydrogen bonding.
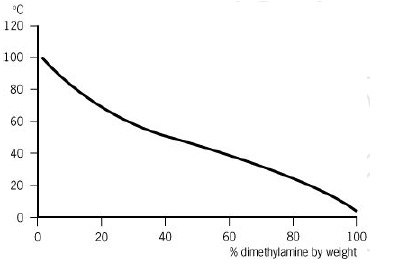 Source: eastman.com
Source: eastman.com
In order to determine the nature of an unknown compound correctly other techniques such as NMR 1 H 13 C HETCOR DEPT Mass spectrometry the chemical reactivity solubility tests sometimes derivatives and physical properties melting point boiling point refractive index are required as well. Let stand for 2-3 hours in the 5-10 cooling mixture. Another group of amines the biogenic amines ethanolamine histamine tyramine putrescine and cadaverine are found in many products fermented by lactic acid bacteria or in poorly conserved food. The ICSC project is a common undertaking between the World Health Organization WHO and. Academiaedu is a platform for academics to share research papers.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title diethylamine boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.