Diethyl ether boiling point
Home » datasheet » Diethyl ether boiling pointDiethyl ether boiling point
Diethyl Ether Boiling Point. -116 C TCI D3479-116 C Alfa Aesar-116 C OU Chemical Safety Data No longer updated More details-1163 C Jean-Claude Bradley Open Melting Point Dataset 21939-116 C Jean-Claude Bradley Open Melting Point Dataset 13163 15719 6838-116 C Alfa Aesar 16767 33224 38990 40976-178–176 F -1166667–1155556 C Wikidata Q202218-177 F -1161111 C Wikidata Q202218. 56 C 1328 F Boiling point of alcohol. 44 0 1473 240000. Diethyl ether and chromium trioxide react violently at room temperature.
 14 1 Introduction To Ethers An Ether Group From slidetodoc.com
14 1 Introduction To Ethers An Ether Group From slidetodoc.com
Diethyl ether CH 3 CH 2 OCH 2 CH 3 is useful as a solvent pair with ligroin but its boiling point 35 C 95 F is too low to make it a good crystallization solvent unless used with a dry iceacetone bath. Because of its characteristics diethyl ether was widely used in many countries as an anesthetic agent but was then. When these 3 fatty acids are mostly or all. The alcohol and the low-boiling components are distilled off and the nonconverted chloroacetate recovered by distillation. CH 3 CH 2 OH. Ether is a staple of synthetic organic chemistry a very commonly-used solvent.
Harmful in contact with skin Warning Acute toxicity dermalH315 100.
Solid acetyl peroxide in contact with ether or any volatile solvent may explode violently. The result is that butane boils at a temperature at which water freezes and is much lower than diethyl ether. Dioxane C 4 H 8 O 2 is easy to remove from. CH 3 CH 2 OH. In the case of diethyl malonate the reaction is conducted at around 100 C and 18 bar at pH 57. C 4 H 10 O MW 74 is 118 C 244 F.
 Source: tcichemicals.com
Source: tcichemicals.com
CH 3 CH 2 2 NH 2. The alcohol and the low-boiling components are distilled off and the nonconverted chloroacetate recovered by distillation. Ethyl acetate is the ester of ethanol. Diethyl ether is a common solvent for oils gums resins etc. Causes skin irritation Warning Skin corrosionirritationH319 100.
Source: quora.com
Methanol and diethyl ether. It is the most common ether known. The relative polarities of pentane diethyl ether and another common TLC solvent ethyl acetate are shown in the table below. 44 0 1473 240000. -1958 C -3204 F Boiling point of liquid helium.
Source: chemspider.com
Hazards Identification Potential Acute Health Effects. The relative polarities of pentane diethyl ether and another common TLC solvent ethyl acetate are shown in the table below. Trimethylamine CH 3 3 N. Harmful if swallowed Warning Acute toxicity oralH312 8864. AReplacing pentane with ethyl acetate.
 Source: quora.com
Source: quora.com
Diethyl carbonate is used as a solvent such as in erythromycin intramuscular injections. Diethyl ether CH 3 CH 2 2 O. It has been used for a wide range of samples like soils sediments and animal and plant tissues. Diethyl ether 60-29-7 100 Toxicological Data on Ingredients. THF DCM Diethyl ether Other commonly used HBP solvents include NMP N-Methyl-pyrrolidone DMAc Di-Methyl-Acetamide.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
It has been used for a wide range of samples like soils sediments and animal and plant tissues. 647 C 1485 F Boiling point of acetone. CH 3 CH 2 2 NH 2. And we can detect its distinctive sweet mostly. Nitrosyl perchlorate ignites and explodes with diethyl ether.
 Source: britannica.com
Source: britannica.com
The melting point is the temperature at which a solid changes into a liquid. C 4 H 10 O MW 74 is 118 C 244 F. The relative polarities of pentane diethyl ether and another common TLC solvent ethyl acetate are shown in the table below. THF DCM Diethyl ether Other commonly used HBP solvents include NMP N-Methyl-pyrrolidone DMAc Di-Methyl-Acetamide. Methyl tert-butyl ether CH 3 OCCH 3 3 is cheap good replacement for diethyl ether given its higher boiling point 52 C 126 F.
 Source: slidetodoc.com
Source: slidetodoc.com
Alcohol has a higher boiling point than ethers of comparable molecular masses. Many people believe these solvents are difficult or slow to remove. A 5-gram portion in ether detonated while being carried Chem. Ethyl acetate is the ester of ethanol. HOCH 2 CH 2 OH.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
In fact the boiling points of ethers are much closer to those of alkanes with similar molecular weights. CH 3 CH 2 OH. The melting point is the temperature at which a solid changes into a liquid. Articles of Diethyl ether are included as well. Because of its characteristics diethyl ether was widely used in many countries as an anesthetic agent but was then.
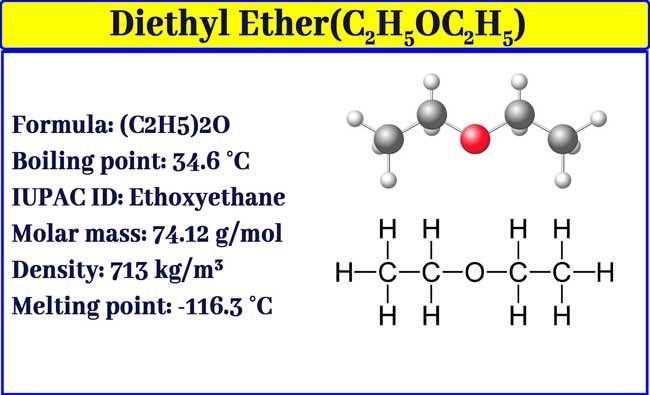 Source: chemistrypage.in
Source: chemistrypage.in
CH 3 CH 2 2 OH. This is actually not the case provided that you do things the right way. Articles of Diethyl ether are included as well. Every organic chemist has used it at one time or another. Diethyl ether is produced by dehydrating ethanol at 300 C in the presence of catalyst.
 Source: youtube.com
Source: youtube.com
Ether is a common name for diethyl ether. It is the most common ether known. 7837 C 1731 F Boiling point of methanol. Articles of Diethyl ether are included as well. Diethyl ether 60-29-7 100 Toxicological Data on Ingredients.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title diethyl ether boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.