Dichloromethane melting point
Home » datasheet » Dichloromethane melting pointDichloromethane melting point
Dichloromethane Melting Point. BUCHIs melting point analyzers fully automate the rather time-consuming process of melting point and boiling point determination and provide highly accurate results. 31290 K at 760 mmHg. Dichloromethane was separated out during the ethanol boiling stage due to its low boiling point 403. Minimize contact with the liquid and handle it under the fume hood.
 Dichloromethane American Chemical Society From acs.org
Dichloromethane American Chemical Society From acs.org
Solvent formula polarity boiling point 0 C water H2O very polar 100 ethanol CH3CH2OH polar 78 methanol CH3OH polar 65 dichloromethane CH2Cl2 slightly polar 40 diethyl ether CH3CH22O slightly polar 35 Organic compounds with one polar functional group and a low number of carbon atoms such. 31290 K at 760 mmHg. Dichloromethane may be harmful if ingested inhaled or absorbed through the skin. Do not exceed the 260oC limit of the thermometer. Dipole moments compare less favorably. 189 and 286 D for dichloromethane and trifluorotoluene respectively.
31290 K at 760 mmHg.
BUCHIs melting point analyzers fully automate the rather time-consuming process of melting point and boiling point determination and provide highly accurate results. This technique selectively dissolves one or more compounds into an appropriate solvent. Solvents like dichloromethane methylene chloride in older literature chloroform diethyl ether or ethyl ester will form two. 12-dichloromethane 250 ethyl acetate 250 methanol 250 3. A drying procedure is therefore necessary to remove all traces of water before the solvent is evaporated. The solution of these dissolved compounds is referred to as the extract.

CHEMICAL PRODUCT AND COMPANY IDENTIFICATION. Product Name Dichloromethane anhydrous Cat No. It is also used as a degreasing agent. Melting PointRange-97 C -1426 F Boiling PointRange 39 C 1022 F Flash Point No information available Evaporation Rate No information available Flammability solidgas Not applicable Flammability or explosive limits Upper 23 vol Lower 13 vol Vapor Pressure 350 mbar. DCM is used as a solvent in the food industry and as a paint remover.
 Source: softschools.com
Source: softschools.com
2 has a dipole moment of 193 D and a boiling point of 52C. Hand your sample of. No boiling point at atmospheric conditions pure as. The melting point depends on the pressure. Prepare at least 100 mL of a saturated sodium bicarbonate solution about 10 wv.
BDH-120 Page 1 of 9 1. The melting point of a solid compound is the temperature at which a phase transition from solid to liquid occurs. Physiochemical Properties of the Technical Grade Test Compound Parameter Value Water solubility 20C Deionized Water 1023 mgL pH 4 0972 mgL pH 7 0880 mgL pH 9 0971 mgL Solvent solubility 20C Acetone 3446 0172 gL. Dichloromethane may be harmful if ingested inhaled or absorbed through the skin. The presence of a soluble impurity almost always causes a decrease in the melting point expected for the pure compound and a broadening of the.

The melting point range is defined as the span of temperature from the point at which the crystals first begin to liquefy to the point at which the entire sample is liquid. The melting point range is defined as the span of temperature from the point at which the crystals first begin to liquefy to the point at which the entire sample is liquid. Looking up the literature mp of caffeine prior to measuring the mp will give you an idea of the approximate temperature to be expected. Melting point of DCM-976 0 C. 189 and 286 D for dichloromethane and trifluorotoluene respectively.
 Source: fishersci.co.uk
Source: fishersci.co.uk
3128 K decomposes at 720 C 3975 C 10355 F. Minimize contact with the liquid and handle it under the fume hood. Gilbert and Martin Experimental. In the case of Caffeine extraction from tea powder the solubility of caffeine in water. The melting point reported in the literature is 238oC so you can heat the Thiele tube rapidly at least up to 200oC.
 Source: acs.org
Source: acs.org
No boiling point at atmospheric conditions pure as. 189 and 286 D for dichloromethane and trifluorotoluene respectively. Dichloromethane was separated out during the ethanol boiling stage due to its low boiling point 403. 69 C pure ai 671 C for TGAI at atmospheric pressure Boiling point. 12-dichloromethane 250 ethyl acetate 250 methanol 250 3.
 Source: byjus.com
Source: byjus.com
Prepare at least 100 mL of a saturated sodium bicarbonate solution about 10 wv. In the case of Caffeine extraction from tea powder the solubility of caffeine in water. It is also a temperature at which a solid crystal turns into a liquid. White to beige Odour. BDH-120 Page 1 of 9 1.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The compound is also used in the production of aerosol formulations. Dichloromethane MSDS Number. Associate membership to the IDM is for up-and-coming researchers fully committed to conducting their research in the IDM who fulfill certain criteria for 3-year terms which are renewable. It is also a temperature at which a solid crystal turns into a liquid. The melting point is the highest temperature at which crystallization may occur.
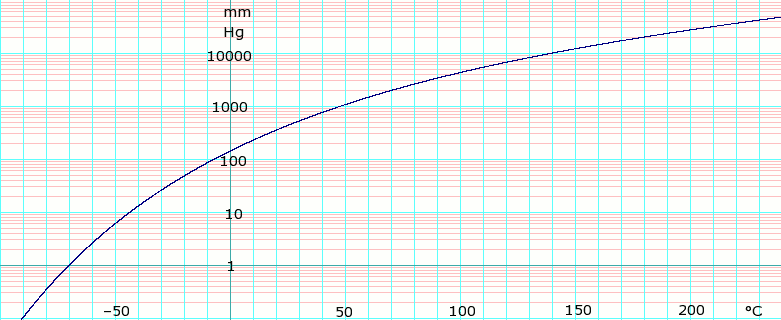 Source: en.wikipedia.org
Source: en.wikipedia.org
Dichloromethane was separated out during the ethanol boiling stage due to its low boiling point 403. News See more News Events. Dichloromethane was separated out during the ethanol boiling stage due to its low boiling point 403. Prolonged exposure to DCM can cause. BUCHI Flux Console and Flux.
 Source: sciencemadness.org
Source: sciencemadness.org
Gilbert and Martin Experimental. CH 2Cl 2 has a dipole moment of 160 D and a boiling point of 40C. Prepare at least 100 mL of a saturated sodium bicarbonate solution about 10 wv. The melting point range is defined as the span of temperature from the point at which the crystals first begin to liquefy to the point at which the entire sample is liquid. Hazards of using Dichloromethane.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title dichloromethane melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.