Dichloromethane boiling point
Home » datasheet » Dichloromethane boiling pointDichloromethane boiling point
Dichloromethane Boiling Point. Decant the liquid from the flask into a 25 ml beaker. This removes the caffeine from the beans but it also removes all flavor. For non-volatile compounds residual solvent is most effectively removed by using the vacuum pump. During the water process method you place the coffee beans in water and heat to around boiling point.
 Dichloromethane Extraction Future4200 From future4200.com
Dichloromethane Extraction Future4200 From future4200.com
97C 207F Boiling Point. The temperature of the column does not have to be above the boiling. 5561 C 10330 F Explosion limits. The boiling point also increases as a result of increasing the size of the halogen as well as increasing the size of the carbon chain. 25 F NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP. Melting point of DCM-976 0 C.
Place one Boileezer into the beaker.
These alcohols form hydrogen bond. The boiling point of a substance is the temperature at which it changes state from liquid to gas throughout the bulk of the liquid. Organic compounds with one polar functional group and a low number of carbon atoms such as methanol ethanol and n-propanol are highly soluble miscible in water. Physical and chemical properties Appearance. The boiling point is specific for the given substanceFor example the boiling point of. Although it is not miscible with water it is polar and miscible with many organic solvents.

Dichloromethane methylene chloride CH 2 Cl 2 is useful as a solvent pair with ligroin but its boiling point 35 C 95 F is too low to make it a good crystallization solvent. 37661 K Solubility in water. With stronger intermolecular attraction of course CH 2F 2 will have a lower boiling point. Aqueous material from the dichloromethane solution at this point. These alcohols form hydrogen bond.

Would not flash Flash point method and additional flammability data are found in Section 5 10. CH 2F 2 is more polar and thus must have stronger binding forces. No data available Melting Point. 37661 K Solubility in water. Would not flash Flash point method and additional flammability data are found in Section 5 10.
 Source: softschools.com
Source: softschools.com
Although it is not miscible with water it is polar and miscible with many organic solvents. Soxhlet extraction is a simple and effective method. Fuels - Densities and Specific Volumes - Densities and specific volumes fuels like anthracite butane gasoil diesel coke oil wood and more. Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. Aqueous material from the dichloromethane solution at this point.
Ethyl Ether 1 VOLATILES. 97C 207F Boiling Point. 12 Vol Upper. Boiling point is the temperature at which the saturated vapor pressure equals the external pressure. Ethyl acetate CH 3 COOC 2 H 5 is an excellent solvent with boiling point 78 C 172 F.
 Source: future4200.com
Source: future4200.com
That CH 2Cl 2 has a higher boiling point. The boiling point at atmospheric pressure. This removes the caffeine from the beans but it also removes all flavor. During the water process method you place the coffee beans in water and heat to around boiling point. At the boiling point molecules anywhere in the liquid may be vaporized.
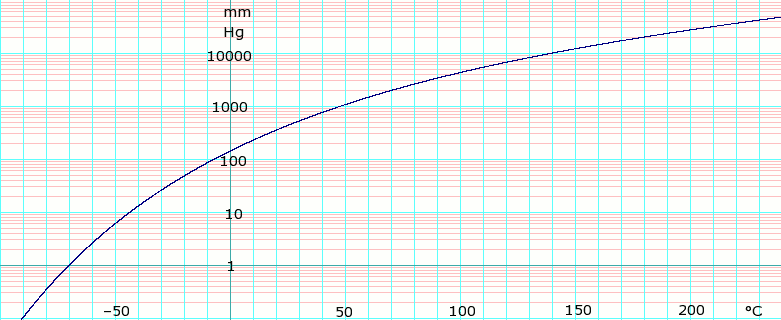 Source: en.wikipedia.org
Source: en.wikipedia.org
CH 2Cl 2 is ionic while CH 2F 2 is molecular. It is also used as a degreasing agent. No data available Melting Point. Place one Boileezer into the beaker. 37661 K Solubility in water.

CH 2Cl 2 has hydrogen-bonding while CH 2F 2 does not. The compound is also used in the production of aerosol formulations. With stronger intermolecular attraction of course CH 2F 2 will have a lower boiling point. The boiling point also increases as a result of increasing the size of the halogen as well as increasing the size of the carbon chain. DCM is used as a solvent in the food industry and as a paint remover.
 Source: acs.org
Source: acs.org
Although it is not miscible with water it is polar and miscible with many organic solvents. -4C 25 F s Ignition temperature. 12 Use the vacuum pump to remove residual solvent. The compound is also used in the production of aerosol formulations. Boiling point is the temperature at which the saturated vapor pressure equals the external pressure.
 Source: en.wikipedia.org
Source: en.wikipedia.org
398-40C 1036-104F Flash Point. 350 mm Hg at 68F 20C VAPOUR DENSITY air 10. Boiling point is the temperature at which the saturated vapor pressure equals the external pressure. CH 2F 2 is more polar and thus must have stronger binding forces. 12 Vol Upper.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Although it is not miscible with water it is polar and miscible with many organic solvents. 97C 207F Boiling Point. Product Name Dichloromethane anhydrous Cat No. It can however be cooled down to 78 C 108 F using a dry. Dichloromethane DCM or methylene chloride is an organochloride compound with the formula C H 2 Cl 2.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title dichloromethane boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.