Decane boiling point
Home » datasheet » Decane boiling pointDecane boiling point
Decane Boiling Point. 7837 C 1731 F Boiling point of nitrogen. Boiling point and melting point d. For example n-butane has a higher boiling point 05 C 311 F than isobutane 117 C 109 F. Acetic acid anhydride CH 3 COO 2 O.
 4 2 Physical Properties Of Alkanes And The Concept Of Homology Chemistry Libretexts From chem.libretexts.org
4 2 Physical Properties Of Alkanes And The Concept Of Homology Chemistry Libretexts From chem.libretexts.org
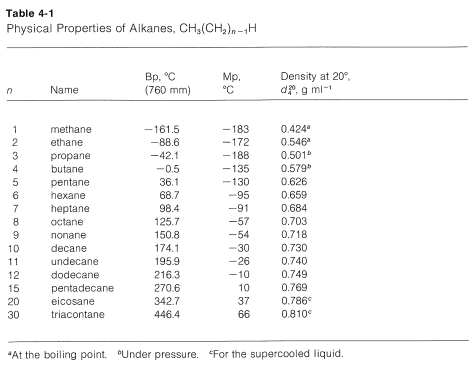
Alkane Molecular Formula Melting Point C Boiling Point C Phase at STP 3 Number of Structural Isomers. For the following compounds write structural formulas and IUPAC names for all possible isomers having the number of the double or triple bond as indicated. A C 4 H 8 one double bond b C 5 H 8 one triple bond. What is the molecular formula of acyclic alkanes. All data Stephenson and Malanowski 1987. Tetramethylbutane CH 3 3 C-CCH 3 3.
C 11 H 24-25.
Alcohol - ethyl grain ethanol C. 56 C 1328 F Boiling point of alcohol. A C 4 H 8 one double bond b C 5 H 8 one triple bond. There is no simple arithmetic relationship between the number of carbon atoms in a formula and the number of isomers. Properties such as melting point and boiling point usually change smoothly and predictably as the number of carbon and hydrogen atoms in the molecules change. C 2 H 6 1833 886.

The number of. The relationship between pressure change and temperature change during evaporation in general. New high-precision vapor pressure data on n-decane n-eicosane and n-octacosane J. Boiling Points of Alkanes Reminder about Alkanes. 7837 C 1731 F Boiling point of methanol.
 Source: chem.libretexts.org
Source: chem.libretexts.org
CH 3 CH 2 8 CH 3. The boiling point is specific for the given substanceFor example the boiling point of. C 10 H 22-30. Note that benzene is a liquid in room temperature and pressure. A bottle of benzene.
 Source: eurekaelearning.com
Source: eurekaelearning.com
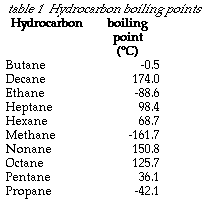
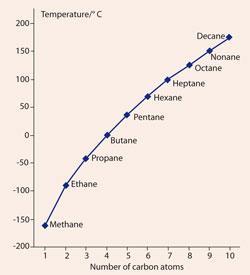
What is the molecular formula of acyclic alkanes. Boiling and freezing temperatures of selected compounds of diesel fuel are listed in Table 2. 5-2-Methylpropyl-decane is the required IUPAC name vii 4-Ethyldeca-1 5 8-triene is the required IUPAC name. Notice that the boiling points of the unbranched alkanes pentane through decane increase rather smoothly with molecular weight but the melting points of the even-carbon chains increase more than those of the odd-carbon chains. C 11 H 24-25.
C 2 H 6 1833 886. Boiling Point 8160 C at 1 atm. What is the molecular formula of acyclic alkanes. 101325 hPa and enthalpy of vaporization molar heat of evaporation then we can estimate the boiling point under another selected pressure. When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils.
 Source: quotesgram.com
Source: quotesgram.com
Acetic acid anhydride CH 3 COO 2 O. For the following compounds write structural formulas and IUPAC names for all possible isomers having the number of the double or triple bond as indicated. What are the two types of alkanes. If we know the boiling point of the substance at some specific pressure tables usually give the value under the so-called normal pressure ie. 7837 C 1731 F Boiling point of methanol.
 Source: edu.rsc.org
Source: edu.rsc.org
C 12 H 26-10. Alcohol - ethyl grain ethanol C. Acetic acid anhydride CH 3 COO 2 O. If we know the boiling point of the substance at some specific pressure tables usually give the value under the so-called normal pressure ie. Boiling point is the temperature at which the saturated vapor pressure equals the external pressure.
 Source: youtube.com
Source: youtube.com
The boiling point is specific for the given substanceFor example the boiling point of. For example n-butane has a higher boiling point 05 C 311 F than isobutane 117 C 109 F. Decane a 10-carbon n-alkane and one of the highest vapor phase constituents of jet propellent-8 was selected to represent the semivolatile fraction for the initial development of a physiologically based pharmacokinetic PBPK model for JP-8Rats were exposed to decane vapors at time-weighted average concentrations of 1200 781 or 273 ppm in a 32-L Leach chamber for 4 hr. 1 Melting Point -457 C 2 Density 077674 gcm 3 at 25C 3 Vapor Density 143 relative air1 Coefficient of Thermal Expansion 0001397 C-1 4 Refractive Index 13442 Na D at 20C 5 Viscosity 0352 cP at 20C 6 Triple Point -438 C 7 Critical Temperature 2724 C 8 Critical Pressure 483 MPa 8 Critical Volume 0173 L Surface Tension 2929 dynecm at. Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight Benzene - Density and Specific Weight - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher.
 Source: chegg.com
Source: chegg.com
Boiling and melting point. Acetic acid anhydride CH 3 COO 2 O. The standard test material for assuring quality control for a lower-temperature flash point apparatus historically has been para-xylene. Determine the double bond stereochemistry E or Z for the following molecules. What is the molecular formula of acyclic alkanes.
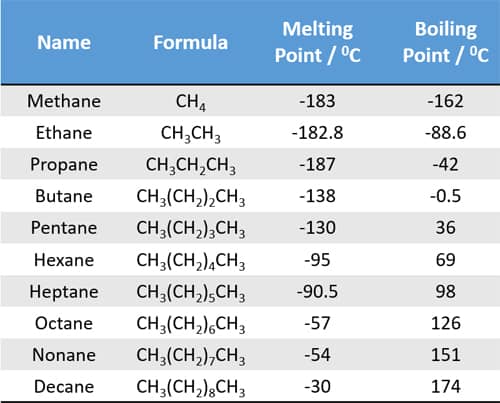 Source: chemistryscl.com
Source: chemistryscl.com
Determine the double bond stereochemistry E or Z for the following molecules. If we know the boiling point of the substance at some specific pressure tables usually give the value under the so-called normal pressure ie. What is the molecular formula of acyclic alkanes. Alkanes are chemical compounds that consist only of the elements carbon C and hydrogen H in proportions according to the general formula. The atoms that form alkanes are linked exclusively by single bonds hence alkanes are saturated hydrocarbons.
 Source: chemsynthesis.com
Source: chemsynthesis.com
3732 K Boiling point of ethanol. A tertiary B quaternary C primary D secondary E none of the above. 1 Melting Point -457 C 2 Density 077674 gcm 3 at 25C 3 Vapor Density 143 relative air1 Coefficient of Thermal Expansion 0001397 C-1 4 Refractive Index 13442 Na D at 20C 5 Viscosity 0352 cP at 20C 6 Triple Point -438 C 7 Critical Temperature 2724 C 8 Critical Pressure 483 MPa 8 Critical Volume 0173 L Surface Tension 2929 dynecm at. Solid BOILING POINTS AND STRUCTURES OF HYDROCARBONS. E Molecular Structure and Spectra 1.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title decane boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.