Cyclopentane boiling point
Home » datasheet » Cyclopentane boiling pointCyclopentane boiling point
Cyclopentane Boiling Point. A second example in which an aldehyde is similarly reduced to a methyl group also illustrates again the use of an acetal protective group. It may be used for simultaneous measurements however interactions between analytes may reduce breakthrough volumes and change desorption efficiencies. PropsSI D P 1e5 Q 0 fluid Same for saturated vapor vG. It occurs as a colorless liquid with a petrol-like odor.
Why Do Cycloalkanes Have A High Boiling Point As Compared To Straight Chain Alkanes Quora From quora.com
4 Spectral Information Expand this section. This method is intended for determining the OSHA-regulated hydrocarbons included within the boiling point range of n-pentane through n-octane. Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight Benzene - Density and Specific Weight - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher. Boiling point is the temperature at which the saturated vapor pressure equals the external pressure. Branching in alkanes increases the distance between molecules and the chains of carbon atoms are less able to come close to one another. In S N 2 mechanism reactivity depends upon the steric hindrance around the C-atom.
CH 3 2 CHCl isopropylchloride or 2-chloropropane ClCH 2 CH 2 CH 3 1 -chloropropane ClCH 2 CH 2 CH 2 CH 3 1-chlorobutane 107.
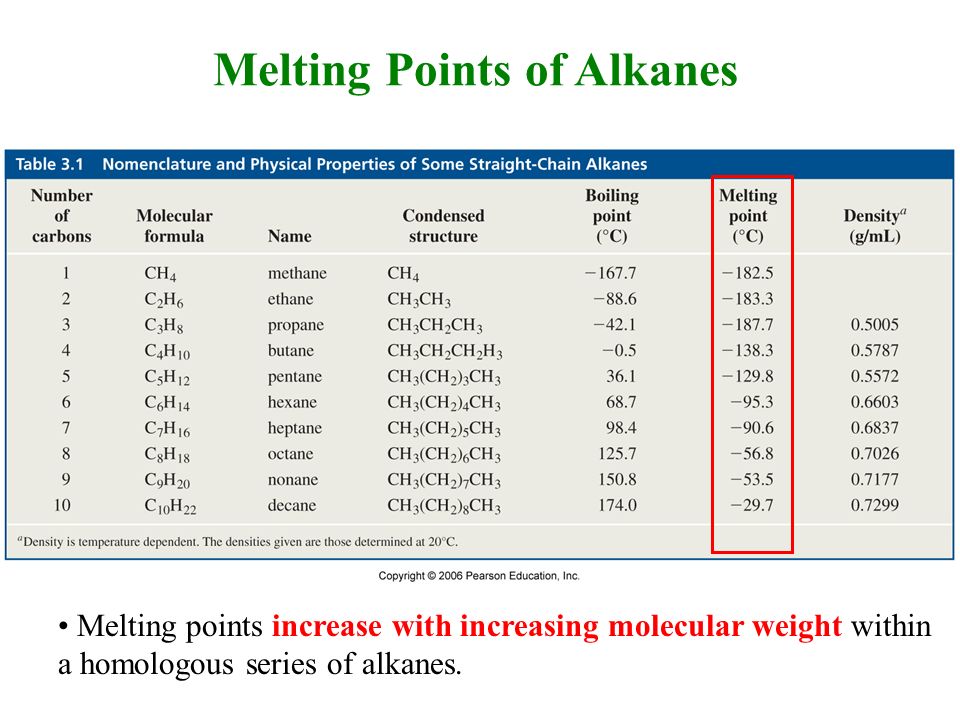
When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils. The overall precisionRSD is 006 over the range of 13 to 53 mg. Branching in alkanes increases the distance between molecules and the chains of carbon atoms are less able to come close to one another. The mechanism of this. Determine the double bond stereochemistry E or Z for the following molecules. The names formulas and physical properties for a variety of alkanes with the generic formula C n H 2n2 are given in the table below.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Which alkyl halide from the following pairs would you expect to react more rapidly by an S N 2 mechanism. Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. A branched alkane is more compact and has a. Give the IUPAC name for each of the following compounds. Low boiling point high flammability high melting point.
 Source: fireengineering.com
Source: fireengineering.com
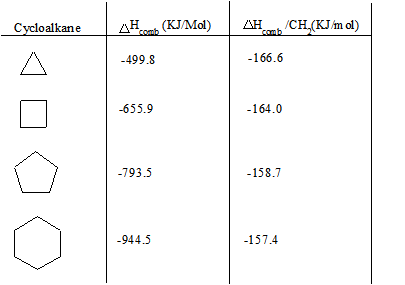
Search azeotropic data of organic mixtures On this page you can check that a mixture of selected organic substances is zeotropic or azeotropicThe azeotropic information boiling pointtemperature composition is predicted using the UNIFAC modified Dortmund version model. Cyclopentane also called C pentane is a highly flammable alicyclic hydrocarbon with chemical formula C 5 H 10 and CAS number 287-92-3 consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. Which alkyl halide from the following pairs would you expect to react more rapidly by an S N 2 mechanism. Branching in alkanes increases the distance between molecules and the chains of carbon atoms are less able to come close to one another. 2989 44 39 Acetic acid.
 Source: researchgate.net
Source: researchgate.net
Search azeotropic data of organic mixtures On this page you can check that a mixture of selected organic substances is zeotropic or azeotropicThe azeotropic information boiling pointtemperature composition is predicted using the UNIFAC modified Dortmund version model. 4 Spectral Information Expand this section. The boiling point is specific for the given substanceFor example the boiling point of. HEATVAPZ HEATVAPZ_T HEATVAPZ_P HEATVALUE Heat of Formation Jmol MEA DEA 22-Iminodiethanol. An azeotrope is a constant-boiling mixture with a constant mole fraction composition of components.
Source: quora.com
PropsSI fluid pcrit Massic volume in m3kg is the inverse of density or volumic mass in kgm3. Acetone CH 3 COCH 3. It may be used for simultaneous measurements however interactions between analytes may reduce breakthrough volumes and change desorption efficiencies. Low boiling point high flammability high melting point. 2040 595 179 40 K f.
Source: chemspider.com
Note that Exercise 5C7 in Atkins10 is P5C5 in Atkins11 and Brief Illustration 5E4 in Atkins10 is 5F3 in Atkins11. Branching in alkanes increases the distance between molecules and the chains of carbon atoms are less able to come close to one another. Boiling point and melting point d. The carbon atoms in saturated hydrocarbons _____. 462 234 1115.
 Source: slideplayer.com
Source: slideplayer.com
Cyclopentane is in the class of. Find more information about azeotropes at Wikipedia Find more information about the method of prediction in the About. Boiling point and melting point d. PropsSI D P 1e5 Q 0 fluid Same for saturated vapor vG. The carbon atoms in saturated hydrocarbons _____.
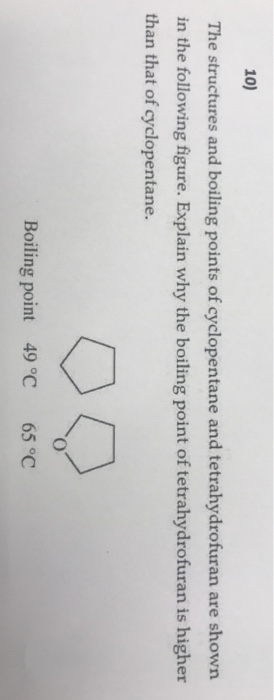
Since it is harder to break apart the bonds in an alcohol the boiling point will be higher. Determine the double bond stereochemistry E or Z for the following molecules. An azeotrope is a constant-boiling mixture with a constant mole fraction composition of components. The US EPA introduces the term polycyclic organic matter POM defined as a class of air toxic compounds with more than one benzene ring and a boiling point of 100C and higher. Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol.
 Source: chem.libretexts.org
Source: chem.libretexts.org
In S N 2 mechanism reactivity depends upon the steric hindrance around the C-atom. Since it is harder to break apart the bonds in an alcohol the boiling point will be higher. The overall precisionRSD is 006 over the range of 13 to 53 mg. Boiling point C K b Ckgmol Freezing point C K f Ckgmol Data source. A high-boiling hydroxylic solvent such as diethylene glycol is commonly used to achieve the temperatures needed.
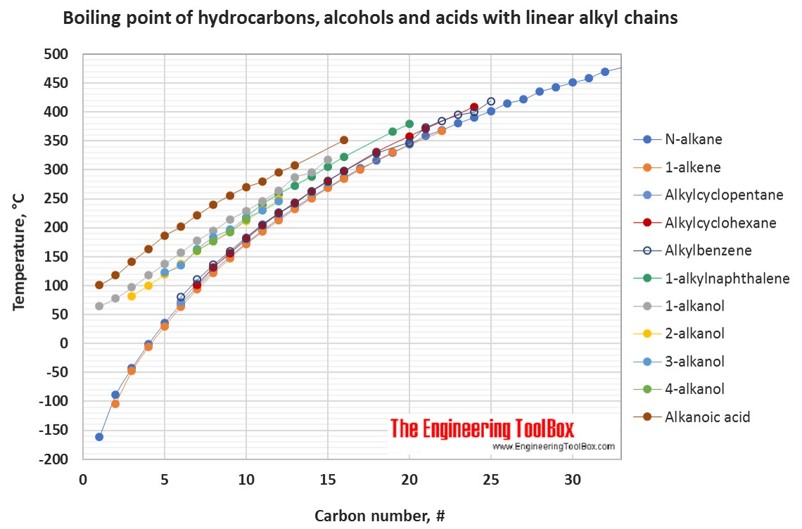 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
The carbon atoms in saturated hydrocarbons _____. A branched alkane is more compact and has a. E Molecular Structure and Spectra 1. 801 265 55 512 K b K f. The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below.
Source: quora.com
Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. Academiaedu is a platform for academics to share research papers. CH 3 2 CHCl isopropylchloride or 2-chloropropane ClCH 2 CH 2 CH 3 1 -chloropropane ClCH 2 CH 2 CH 2 CH 3 1-chlorobutane 107. Acetone CH 3 COCH 3. The boiling points of the alkanes gradually increase with the.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title cyclopentane boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.