Cyclohexene boiling point
Home » datasheet » Cyclohexene boiling pointCyclohexene boiling point
Cyclohexene Boiling Point. Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More. 103 deg C VP exp database. From this data and the results of your. Human Metabolome Database HMDB 323 Solubility.
 1 Methyl 1 Cyclohexene 97 591 49 1 From sigmaaldrich.com
1 Methyl 1 Cyclohexene 97 591 49 1 From sigmaaldrich.com
CRC Press Taylor Francis Boca Raton FL 2005 p. Assemble the reflux apparatus and clamp it securely with a heating mantle underneath. Fit the water in and water out. Coefficents calculated by NIST from authors data. The bromine reagent is a reddish-orange color. It turns out that if you take THF and.
8298 C 18136 F.
The purity of the cyclohexene is determined by gas chromatographic GC analysis and a yield calculated. Briefly identify the important distinctions between a saturated hydrocarbon and an. Log Octanol-Water Partition Coef SRC. -7292 Mean or Weighted MP VPmm Hg25 deg C. Generally flash point increases with an increase in boiling point. 1-pentene or 1- butene 2.
O alcohols require strong acids and significant amounts of heat up to 180. The primary aim of the cards is to promote the safe use of chemicals in the workplace. Slightly soluble in glycerol. Cyclohexane cyclohexene Functional group is an atom or group of atoms which when present in different molecules causes them to have similar chemical properties. The main target users are workers and those responsible for occupational safety and health.
 Source: scbt.com
Source: scbt.com
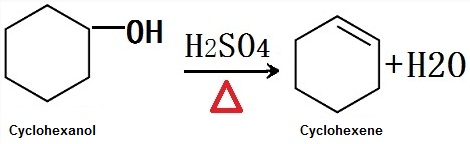
Composition Range TLVLD50LC50 Ethanol Alcohol Denatured 64-17. - cyclohexanol dehydrated to form cyclohexene in elimination reaction - proton catalyst used -OH bad leaving group to protonate alcohol water lost to form carbocation deprotonation forms cyclohexene - Azeotrope- mixture of two liquids that has a single sharp boiling point - Minimum boiling point azeotropes- boils at a temperature below that of either pure liquid - azeotrope will continue. Find more information about azeotropes at Wikipedia Find more information about the method of prediction in the About. That the boiling points of straight-chain alkenes increase with increasing molar mass. Fit the water in and water out.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Mole of alkene in a 11 ratio and 1 mole of water reference 1. Chemical Name Common Name CAS No. Find more information about azeotropes at Wikipedia Find more information about the method of prediction in the About. The bromine reagent is a reddish-orange color. From this data and the results of your.

1-pentene or 1- butene 2. Dont forget a boiling stone. The formation of a heavy brown precipitate MnO 2 is a positive test for unsaturation. The term dehydrate means to remove water and is used to identify the nature of the atomsmolecules eliminated to form the double bond of the alkene in our product. -7292 Mean or Weighted MP VPmm Hg25 deg C.
 Source: chegg.com
Source: chegg.com
Human Metabolome Database HMDB 323 Solubility. Human Metabolome Database HMDB 323 Solubility. Organic systems Toluene ILs Heptane and Cyclohexane ILs Cyclohexene For organic systems the solubility was determined by binodal curve data using the cloud-point method as well described elsewhere Binodal curves were obtained by the titration and turbidimetry method which consists of visual observation of permanent turbidity cloud. This means the intermolecular forces between unsaturated hydrocarbon molecules are dominantly weak Van der Waals force. Briefly identify the important distinctions between a saturated hydrocarbon and an.
 Source: chemicalbook.com
Source: chemicalbook.com
They show a gradual change in physical properties eg. The Good Scents Company Information System. 3046 kJmole at boiling point. Boiling point butane-05 1-butene-62 1-butyne 80 5 Melting point pentane-130 1-pentene-1652 1-pentyne-900 Boiling point pentane 36 1-pentene 299 1-pentyne 401 Just like their saturated counterparts the unsaturated hydrocarbons are usually non-polar. Alcohols are frequently converted into the desired alkene using an acid catalyzed Elimination reaction.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Magnetic susceptibility χ-57510 6 cm 3 mol Refractive index n D 14465 Hazards Safety data sheet. Human Metabolome Database HMDB 323 Solubility. The surrounding temperature around a storage tank should always. Flash point is an important parameter for safety considerations especially during storage and transportation of volatile petroleum products ie LPG light naphtha gasoline in a high-temperature environment. Put 05ml of the product under test into a test tube.
 Source: chemsynthesis.com
Source: chemsynthesis.com
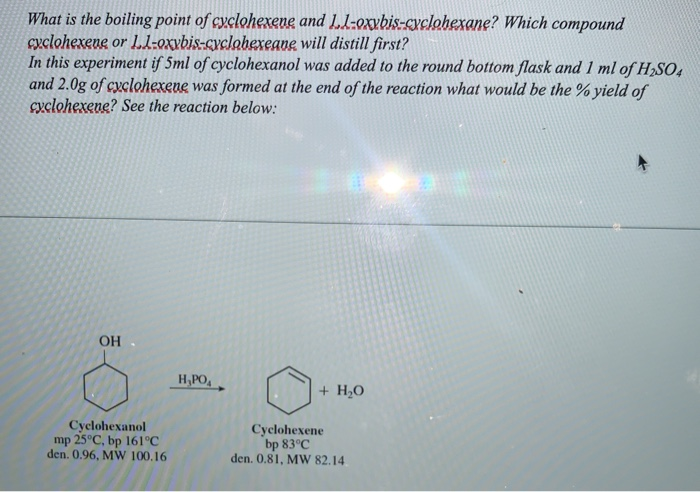
The main target users are workers and those responsible for occupational safety and health. Fr om a 60 mL of cyclohexanol 229 grams of cyclo-hexene is produced. From that 60 m L cyclohexanol. Sulfur exists in many forms with the general molecular formula Sn. In the fumehood measure out 06 mL of 1-decene 185 mL of cyclohexene and place it into a round bottom flask.

The distillate is collected at boiling temperature range of 77 C to 80C. 34-dimethoxybenzaldehyde cyclohexene hexane MeMe In Part C of the lab take home once you have correctly identified the functional group present in your unknown compounds your TA will provide you with the 1H NMR and 13C NMR spectra for your compounds as well as the compounds molecular formula. This means the intermolecular forces between unsaturated hydrocarbon molecules are dominantly weak Van der Waals force. Sulfur exists in many forms with the general molecular formula Sn. Boiling Point C Feature.
 Source: chegg.com
Source: chegg.com
Has the higher boiling point. Miscible with organic solvents Vapor pressure. 3347 kJmole at 25 C Lide DR. T heoretically in a dehydration reaction one mole of alcohol produces one. Composition Range TLVLD50LC50 Ethanol Alcohol Denatured 64-17.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title cyclohexene boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.