Cyclohexane melting point
Home » datasheet » Cyclohexane melting pointCyclohexane melting point
Cyclohexane Melting Point. Branched —more sphere-like - - lower surface area — lower boiling point. Carbon dioxide and carbon monoxide may form when heated to decomposition. The deviation of bond angle in cyclohexane molecules is more than in cyclopentane it should be more strained and less reactive than cyclopentane. 1 Structures Expand this section.
 Essential Organic Chemistry Ppt Download From slideplayer.com
Essential Organic Chemistry Ppt Download From slideplayer.com
We say that such a body melts. Calibration Compound Literature Melting Point C Actual Melting Point C 3 Phenylpropionic Acid 486 43-45 Acetamide 823 69-71 Benzamide 133 103-117 Ice 0 3 Table 6. The melting and freezing point changes with pressure but normally they are given at 1 atm. But actually it is less strained and more stable than. 2 Names and Identifiers Expand this section. Cyclohexane is a colorless flammable liquid with a distinctive detergent-like odor reminiscent of cleaning products.
Carbon dioxide and carbon monoxide may form when heated to decomposition.
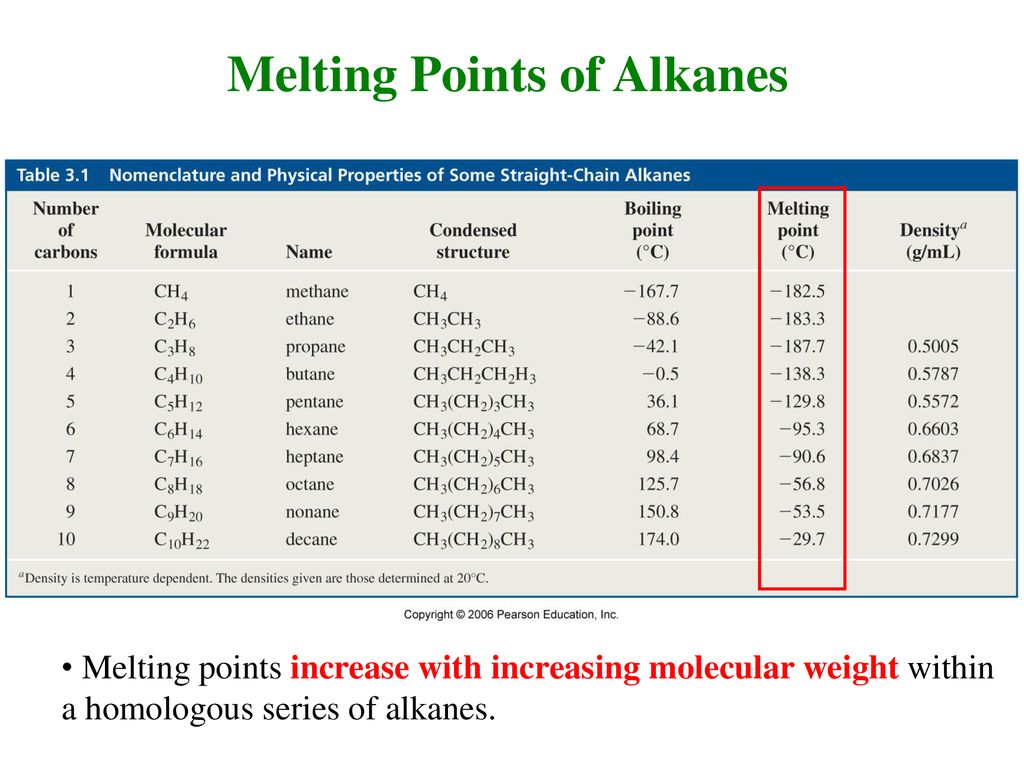
The boiling and melting point of alkane depends upon the length of the carbon chain. Most pure organic compounds melt over a narrow temperature range of 1-2 C. The low-temperature below 186 K phase II is ordered. The high-temperature phase I stable between 186 K and the melting point 280 K is a plastic crystal which means the molecules retain some rotational degree of freedom. The presence of a soluble impurity almost always causes a decrease in the melting point expected for the pure compound and a broadening of the. The low melting and boiling points of covalent compounds can be explained as below.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
It is also a temperature at which a solid crystal turns into a liquid. 2 Names and Identifiers Expand this section. A solution is prepared by dissolving 05580g of an unknown solute in 3350 g cyclohexane. Nuclear Magnetic Resonance Line-Shape and Double-Resonance Studies of Ring Inversion in Cyclohexane-d 11 A. The melting point depends on the pressure.

Thus a melting point reflects the thermal energy needed to convert the highly ordered array of molecules in a crystal lattice to the randomness of a liquid. Lab 7 - Determination of the Molar Mass of an Unknown Solid by Freezing Point Depression Goal and Overview In the first part of the lab a series of solutions will be made in order to determine the freezing point depression constant K f for cyclohexaneThe freezing points of these solutions which will contain known amounts of p-dichlorobenzene dissolved in cyclohexane will be measured. Alkanes comprising of more than three carbon atoms are capable of. In this equation T FP is the freezing point depression the change in the freezing point that occurs when the solute dissolves in the solvent – and k f is the molal freezing point depression constant for the solvent. The deviation of bond angle in cyclohexane molecules is more than in cyclopentane it should be more strained and less reactive than cyclopentane.
 Source: chemsynthesis.com
Source: chemsynthesis.com
2 Names and Identifiers Expand this section. Page 5 of 7 MSDS Cyclohexane Vapor Pressure mm Hg. Branched —more sphere-like - - lower surface area — lower boiling point. In the following diagram cyclohexane represents a low-energy reference point. Two other low-temperature metastable phases III.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Calibration Compound Literature Melting Point C Actual Melting Point C 3 Phenylpropionic Acid 486 43-45 Acetamide 823 69-71 Benzamide 133 103-117 Ice 0 3 Table 6. Most pure organic compounds melt over a narrow temperature range of 1-2 C. A similar equation can be written to describe what happens to the freezing point or melting point of a solvent when a solute is added to the solvent. Molecular size is important but shape is also. Branched —more sphere-like - - lower surface area — lower boiling point.
But actually it is less strained and more stable than. 41 to 61 C 106 to 142 F. The melting point depends on the pressure. Compound Melting Point C. 3 Chemical and Physical Properties Expand this section.
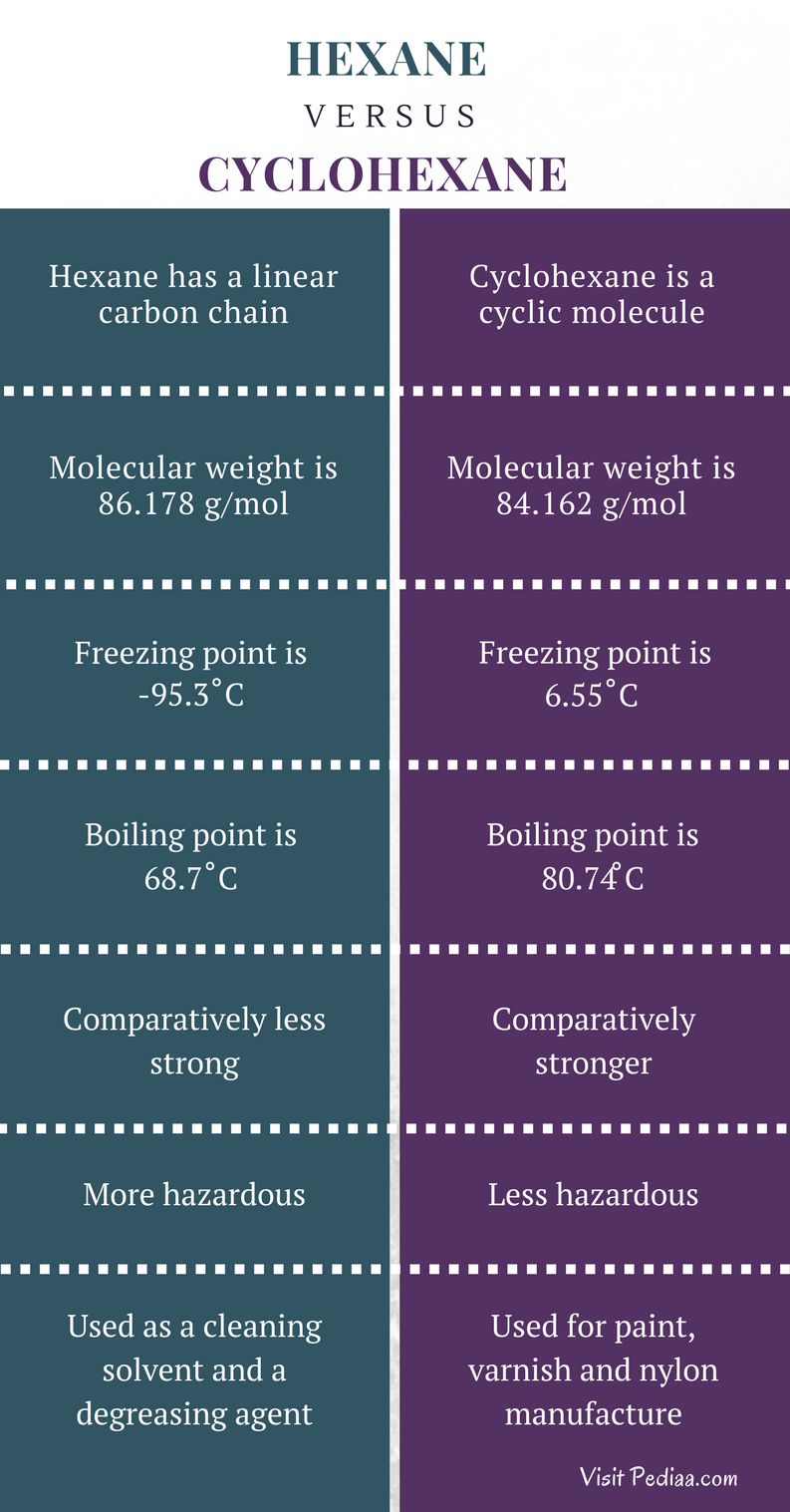 Source: pediaa.com
Source: pediaa.com
The point of this post is to describe. Trans-cyclohexane-12-diol is a cyclohexane-12-diol with trans-configuration. The boiling and melting point of alkane depends upon the length of the carbon chain. Branched —more sphere-like - - lower surface area — lower boiling point. The melting point is the highest temperature at which crystallization may occur.

A pure substance has the same freezing and melting points in practice a small difference between these quantities can be observed. If the length is more the boiling melting point will be higher. Melting points of various compounds determined experimentally. Tf Kf m molality Tf 218 C 0109 mole solutekg Kf. It is a di-substituted derivative of cyclohexane and is classified as a diol meaning that it has two OH functional groups.
 Source: ch.imperial.ac.uk
Source: ch.imperial.ac.uk
2 Names and Identifiers Expand this section. Calculate the molar mass of the unknown solute. Tf Kf m molality Tf 218 C 0109 mole solutekg Kf. The melting point depends on the pressure. The boiling and melting point of alkane depends upon the length of the carbon chain.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Bourn Journal of the American Chemical Society 1967 89 4 760-768 DOI. 4 Spectral Information Expand this section. Thus a melting point reflects the thermal energy needed to convert the highly ordered array of molecules in a crystal lattice to the randomness of a liquid. Its prevalence undoubtedly a consequence of its stability makes it the most important of the cycloalkanes. 2 Names and Identifiers Expand this section.
 Source: slideplayer.com
Source: slideplayer.com
The melting point is specific for a given substance. Alkanes consist of four carbon atoms at standard temperatures. Its prevalence undoubtedly a consequence of its stability makes it the most important of the cycloalkanes. Page 5 of 7 MSDS Cyclohexane Vapor Pressure mm Hg. Calibration Compound Literature Melting Point C Actual Melting Point C 3 Phenylpropionic Acid 486 43-45 Acetamide 823 69-71 Benzamide 133 103-117 Ice 0 3 Table 6.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title cyclohexane melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.