Clinical trial empirical endpoint support
Home » datasheet » Clinical trial empirical endpoint supportClinical trial empirical endpoint support
Clinical Trial Empirical Endpoint Support. Further secondary endpoints were clinical early and late responses in predefined subgroups eg by sex age and bacteraemia at baseline These endpoint parameters are further defined in the appendix p 11. 10 to 62 points and 43 points 95 CI. It describes the process of undertaking an assessment using the RoB 2 tool summarizes the important issues for each domain of bias and ends with a list of the key differences between RoB 2. Participants 92 aircraft passengers aged 18 and over were screened for participation.
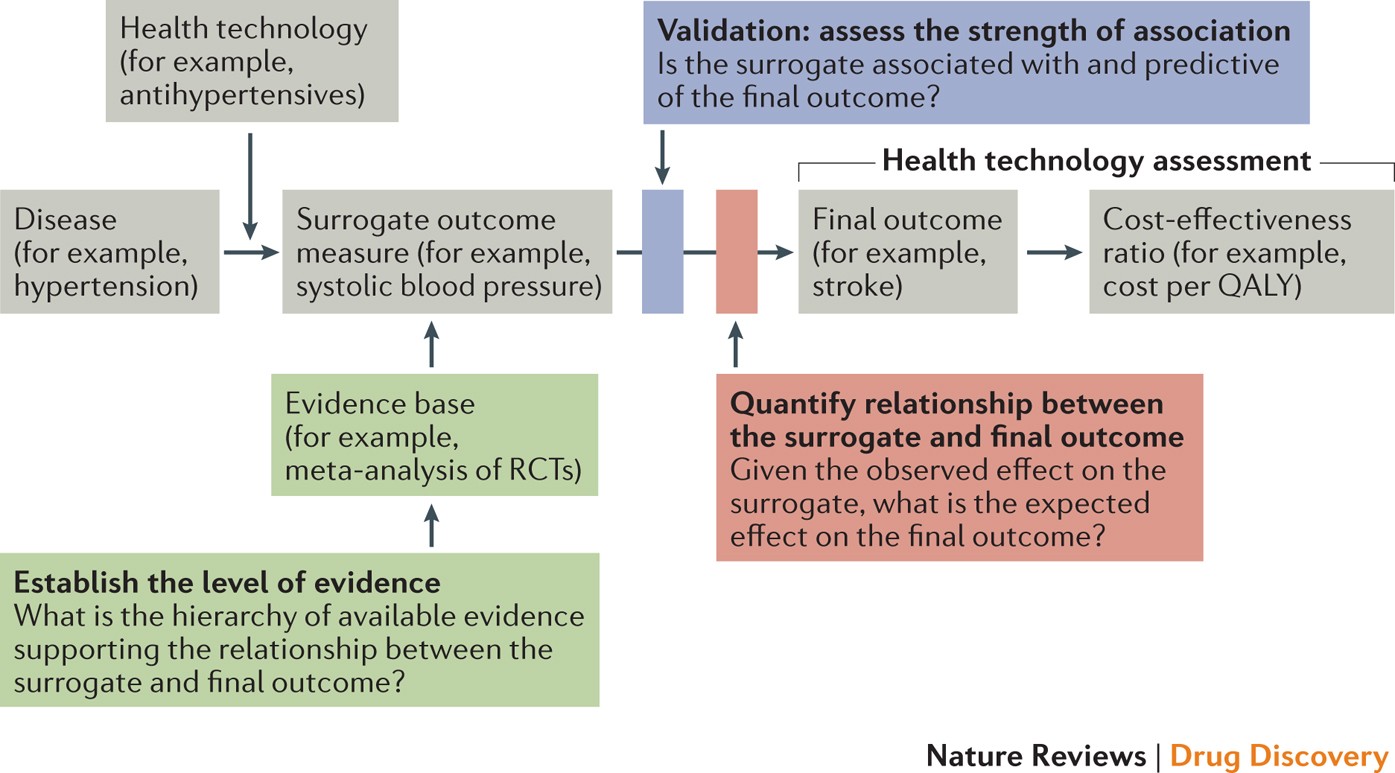 Use Of Surrogate End Points In Healthcare Policy A Proposal For Adoption Of A Validation Framework Nature Reviews Drug Discovery From nature.com
Use Of Surrogate End Points In Healthcare Policy A Proposal For Adoption Of A Validation Framework Nature Reviews Drug Discovery From nature.com
Objective To determine if using a parachute prevents death or major traumatic injury when jumping from an aircraft. It describes the process of undertaking an assessment using the RoB 2 tool summarizes the important issues for each domain of bias and ends with a list of the key differences between RoB 2. Four short-term trials and one maintenance trial in adult patients and one short-term trial in adolescents ages 13 to 17 years with schizophrenia see Clinical Studies 141 Four short-term monotherapy trials and one 6-week adjunctive trial in adult patients and one short-term monotherapy trial in pediatric patients ages 10 to 17 years with manic or mixed episodes see Clinical Studies. Clinical trials are carefully designed. The protocol 1 for conducting the trial and the statistical analysis plan SAP detailing the planned data analyses are developed well before the first participant is enrolled. All drug and many device trials target a subset of the population meaning not.
A clinical trial might also include an extended post-study follow-up period from months to years for people who have participated in the trial a so-called extension phase which aims to identify long-term impact of the treatment.
These domains were identified based on both empirical evidence and theoretical considerations. Moreover 29 vs 21 met the primary endpoint in the piperacillin-tazobactam and meropenem arms respectively risk difference 8 95 CI 11 to 28 which. Losartan potassium is a white to off-white free-flowing crystalline powder with a molecular weight of 46101. Blinding is a measure in randomized controlled trials RCT to reduce detection and performance bias. Therefore the timelines for submitting and posting clinical trial results information for applicable device clinical trials for unapproved or uncleared devices in proposed 1144 and 1152 respectively could result in the public availability of clinical trial results information for such trials before the information submitted during registration is posted in accordance with proposed. Because of the physical component of interventions blinding is not easily applicable in surgical trials.
 Source: sciencedirect.com
Source: sciencedirect.com
High quality protocols facilitate proper conduct reporting and external review of clinical trials. Evidence-based medicine EBM is the conscientious explicit and judicious use of current best evidence in making decisions about the care of individual patients The aim of EBM is to integrate the experience of the clinician the values of the patient and the best available scientific information to guide decision-making about clinical management. However the completeness of trial protocols is often inadequate. Further secondary endpoints were clinical early and late responses in predefined subgroups eg by sex age and bacteraemia at baseline These endpoint parameters are further defined in the appendix p 11. Because of the physical component of interventions blinding is not easily applicable in surgical trials.
Source:
Moreover 29 vs 21 met the primary endpoint in the piperacillin-tazobactam and meropenem arms respectively risk difference 8 95 CI 11 to 28 which. The PRINCIPLE trial platform is led from the Primary Care and Vaccines Collaborative Clinical Trials Unit at the University of Oxfords Nuffield Department of Primary Care Health Sciences. There is evidence that lack of blinding leads to overestimated treatment effects. This trial has generated preliminary data directed toward a definitive randomized clinical trial. Therefore the timelines for submitting and posting clinical trial results information for applicable device clinical trials for unapproved or uncleared devices in proposed 1144 and 1152 respectively could result in the public availability of clinical trial results information for such trials before the information submitted during registration is posted in accordance with proposed.
 Source: ccforum.biomedcentral.com
Source: ccforum.biomedcentral.com
Oxidation of the 5-hydroxymethyl group on the imidazole ring. However the completeness of trial protocols is often inadequate. The protocol 1 for conducting the trial and the statistical analysis plan SAP detailing the planned data analyses are developed well before the first participant is enrolled. In that study Butler et al. The definitions of these endpoints are critical and should be scrutinized carefully to ensure that they are not too lenient.
 Source: urotoday.com
Source: urotoday.com
Further secondary endpoints were clinical early and late responses in predefined subgroups eg by sex age and bacteraemia at baseline These endpoint parameters are further defined in the appendix p 11. Four short-term trials and one maintenance trial in adult patients and one short-term trial in adolescents ages 13 to 17 years with schizophrenia see Clinical Studies 141 Four short-term monotherapy trials and one 6-week adjunctive trial in adult patients and one short-term monotherapy trial in pediatric patients ages 10 to 17 years with manic or mixed episodes see Clinical Studies. Trial design and registration. The biggest barrier to completing studies is the shortage of people who take part. This chapter summarizes the main features of RoB 2 applied to individually randomized parallel-group trials.
 Source: pharmrev.aspetjournals.org
Source: pharmrev.aspetjournals.org
MSAC appraises new medical services proposed for public funding and provides advice to Government on whether a new medical service should be publicly funded and if so its circumstances on an assessment of its comparative safety clinical effectivenesscost-effectiveness and total cost using the best available evidence. Is defined as the number of days that a patient is alive and free of organ support through the first 21 days after trial entry. Therefore the timelines for submitting and posting clinical trial results information for applicable device clinical trials for unapproved or uncleared devices in proposed 1144 and 1152 respectively could result in the public availability of clinical trial results information for such trials before the information submitted during registration is posted in accordance with proposed. Clinical trials are carefully designed. 02 to 04 points greater KCCQ overall summary scores than did patients reporting no change in their heart failure status at 4 and 24 weeks after randomization in a clinical trial of iron supplementation in HFrEF.
 Source: nature.com
Source: nature.com
Further secondary endpoints were clinical early and late responses in predefined subgroups eg by sex age and bacteraemia at baseline These endpoint parameters are further defined in the appendix p 11. Four short-term trials and one maintenance trial in adult patients and one short-term trial in adolescents ages 13 to 17 years with schizophrenia see Clinical Studies 141 Four short-term monotherapy trials and one 6-week adjunctive trial in adult patients and one short-term monotherapy trial in pediatric patients ages 10 to 17 years with manic or mixed episodes see Clinical Studies. Know the risks and potential benefits of clinical studies and talk to your health care provider. Moreover 29 vs 21 met the primary endpoint in the piperacillin-tazobactam and meropenem arms respectively risk difference 8 95 CI 11 to 28 which. 44 The document first lays out a framework that aims to align the clinical study objective.
 Source: ctti-clinicaltrials.org
Source: ctti-clinicaltrials.org
02 to 04 points greater KCCQ overall summary scores than did patients reporting no change in their heart failure status at 4 and 24 weeks after randomization in a clinical trial of iron supplementation in HFrEF. The protocol and the SAP constitute some of the most important metadata of the trial. Moreover 29 vs 21 met the primary endpoint in the piperacillin-tazobactam and meropenem arms respectively risk difference 8 95 CI 11 to 28 which. Generally this should include no more than 10 - 20 key references that demonstrate a thorough review of the literature and provide support for the methodology dosage choice measurement techniques etc. Four short-term trials and one maintenance trial in adult patients and one short-term trial in adolescents ages 13 to 17 years with schizophrenia see Clinical Studies 141 Four short-term monotherapy trials and one 6-week adjunctive trial in adult patients and one short-term monotherapy trial in pediatric patients ages 10 to 17 years with manic or mixed episodes see Clinical Studies.
 Source: trialsjournal.biomedcentral.com
Source: trialsjournal.biomedcentral.com
However the completeness of trial protocols is often inadequate. However the completeness of trial protocols is often inadequate. The definitions of these endpoints are critical and should be scrutinized carefully to ensure that they are not too lenient. All drug and many device trials target a subset of the population meaning not. It is freely soluble in water soluble in alcohols and slightly soluble in common organic solvents such as acetonitrile and methyl ethyl ketone.
 Source: nature.com
Source: nature.com
Footnotes Aetna considers non-elastic leg binders eg CircAid LegAssist Reid Sleeve medically necessary for members who meet the selection criteria for pressure gradient support stockings listed above. Its empirical formula is C 22 H 22 ClKN 6 O and its structural formula is. Because of the physical component of interventions blinding is not easily applicable in surgical trials. This chapter summarizes the main features of RoB 2 applied to individually randomized parallel-group trials. Enrichment Strategies for Clinical Trials to Support Determination of Effectiveness of Human Drugs and Biological Products.
 Source: pharmaintelligence.informa.com
Source: pharmaintelligence.informa.com
Its empirical formula is C 22 H 22 ClKN 6 O and its structural formula is. It describes the process of undertaking an assessment using the RoB 2 tool summarizes the important issues for each domain of bias and ends with a list of the key differences between RoB 2. Clinical trials are carefully designed. The biggest barrier to completing studies is the shortage of people who take part. Exploratory endpoints were differences in antibiotic resistance and microbial load of each species in faecal isolates at discontinuation of study drug and after oral step-down therapy.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title clinical trial empirical endpoint support by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.