Chloroform boiling point
Home » datasheet » Chloroform boiling pointChloroform boiling point
Chloroform Boiling Point. Water butanoles propanoles aniline toluene bromoform dimethylformamide. Direction of Heat. Deuterated chloroform is by far the most common solvent used in NMR spectroscopy. Not determined Vapor density.
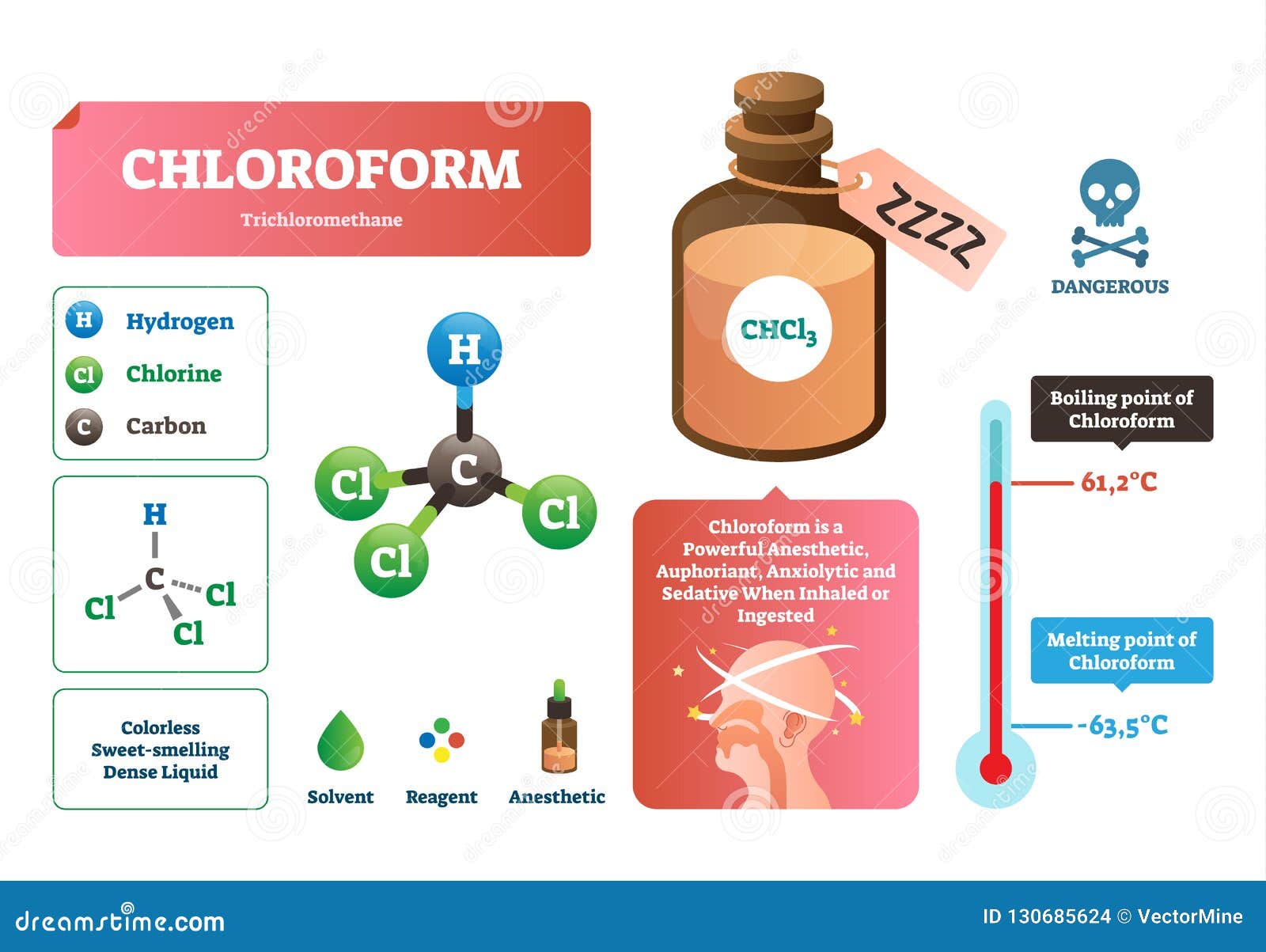 Chloroform Vector Illustration Chemical Liquid Structure Characteristics Stock Vector Illustration Of Chain Chcl3 130685624 From dreamstime.com
Chloroform Vector Illustration Chemical Liquid Structure Characteristics Stock Vector Illustration Of Chain Chcl3 130685624 From dreamstime.com
61 C 1013 hPa Density. 23 K to 353. Since most of the extractions are performed using aqueous solutions ie 5 NaOH 5 HCl the miscibility of the solvent with water is a crucial point as well as the compatibility of the reagent with the compounds and the solvent of the solution to be extracted. 213 mbar 20 C Odor threshold. Dioxane methanol ethanol nitric acid nitromethane pyridine phosphorous oxychloride. Boiling point of pure water increases with increase in pressure.
The degree of dissociation of the solute and the molar mass of the solute can be calculated with the help of the boiling point elevation formula.
1 Let us use the Clausius-Clapeyron Equation. Bromine chloroform cyclohexane ethyl acetate triethylamine. LD50 Rabbit 3980. Ph Eur - Find MSDS or SDS a COA data sheets and more information. 412 Air 10 pH-value. Both binary and ternary systems containing ionic liquid present.
 Source: ddbst.com
Source: ddbst.com
Energy Forms. The degree of dissociation of the solute and the molar mass of the solute can be calculated with the help of the boiling point elevation formula. -1958 C -3204 F Boiling point of liquid helium. Water butanoles propanoles aniline toluene bromoform dimethylformamide. 1 kg of the given solution contains 0035kg of NaCl and 0965kg of H 2 O.
 Source: researchgate.net
Source: researchgate.net
23 K to 353. Direction of Heat. For example phenol molecular weight MW 94 boiling point bp 182 C 3596 F has a boiling point more than 70 degrees higher than that of toluene C 6 H 5 CH 3. Deuterated chloroform is an isotopologue of chloroform with a single deuterium atom. Deuterated chloroform is by far the most common solvent used in NMR spectroscopy.
 Source: dreamstime.com
Source: dreamstime.com
Notice that the. This decrease will affect the time it takes to cook anything in water to the extent that any food that requires five minutes to prepare at. Water boils at 373 K and. Boiling Points for common Liquids and Gases - Boiling temperatures for some common liquids and gases - acetone. 3732 K Boiling point of ethanol.
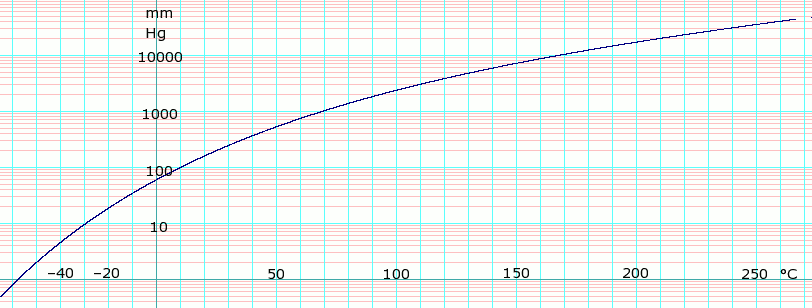 Source: en.wikipedia.org
Source: en.wikipedia.org
For carbon tetrachloride the boiling point constant is 503 Cm and the boiling point of pure carbon tetrachloride is 7650 C. Slightly soluble Boiling pointBoiling range. The boiling point observed for the water-methanol mixture was much higher than that of pure methanol since water has a much higher boiling point and lower vapor pressure than methanol. Both binary and ternary systems containing ionic liquid present. The change in the boiling point is calculated from.
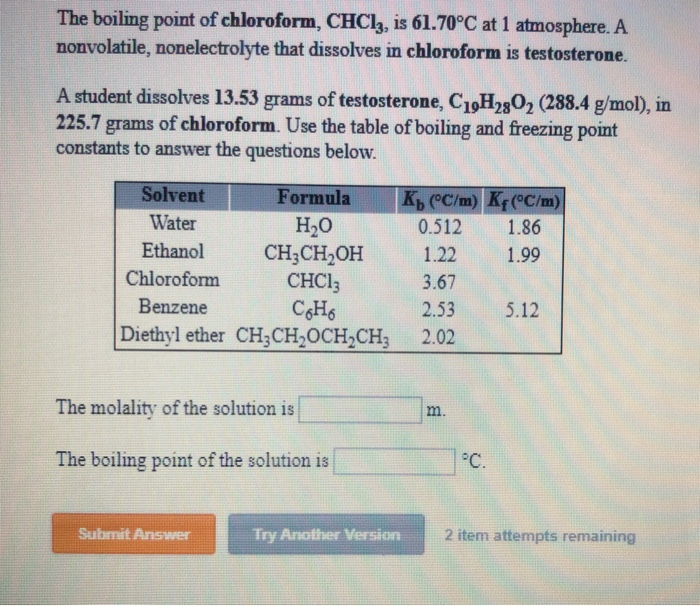 Source: chegg.com
Source: chegg.com
Chloroform is a colorless volatile liquid derivative of trichloromethane with an ether-like odor. Where K b is the molal boiling point constant and m is the concentration of the solute expressed as molality. 1 Physical and chemical properties Physical state Molecular weight pH Critical temperature Relative density Vapor pressure Vapor density Volatility Odor threshold Evaporation rate Solubility Odor Color Molecular formula CHCl3 VOC 100. The product is stable. LD50 Rabbit 3980.
 Source: doubtnut.app
Source: doubtnut.app
For example the boiling point of pure water at standard atmospheric pressure or sea level is 100C 212F while at 10000 feet 3048m it is 9039 C 1947F. LD50 Rat 695 mgkg. The change in the boiling point is calculated from. Direction of Heat. Where K b is the molal boiling point constant and m is the concentration of the solute expressed as molality.
 Source: thermopedia.com
Source: thermopedia.com
Δ T b K b m. The quantity of heat required to completely vaporise a unit mass of a liquid gas at its boiling point is called latent heat of vaporisation of the liquid. Where K b is the molal boiling point constant and m is the concentration of the solute expressed as molality. 1 kg of the given solution contains 0035kg of NaCl and 0965kg of H 2 O. Δt i K b.
 Source: toppr.com
Source: toppr.com
CDCl 3 is a common solvent used in NMR spectroscopyDeuterochloroform is produced by the haloform reaction citation needed the reaction of acetone or ethanol with sodium hypochlorite or calcium hypochlorite. Slightly soluble Boiling pointBoiling range. MW 92 bp 111 C 2318 F. Not determined Relative density. Similarly an azeotropic mixture that has a boiling point lesser than its constituents is known as minimum boiling azeotropes.
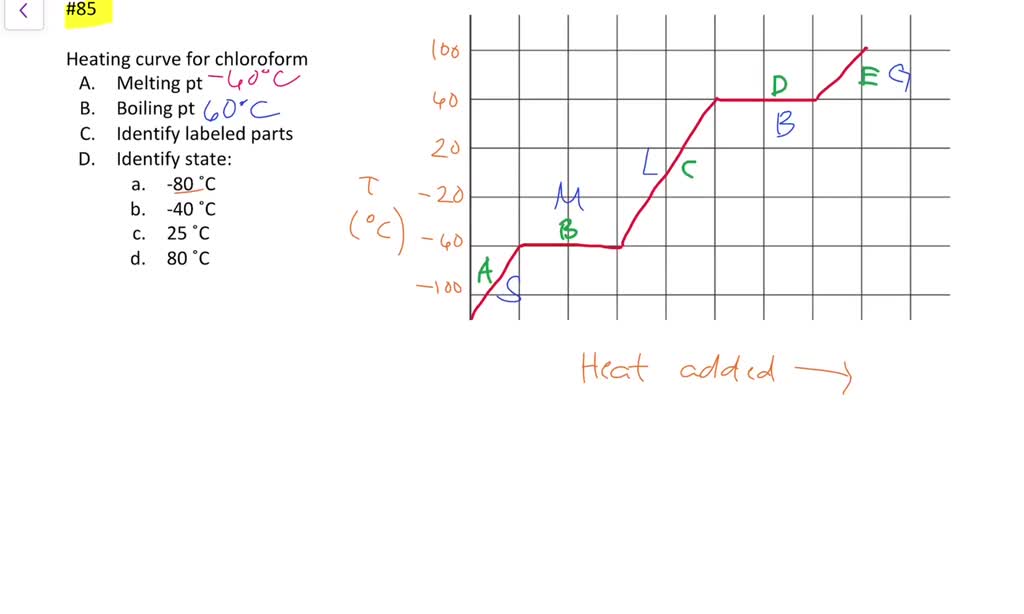 Source: numerade.com
Source: numerade.com
For example phenol molecular weight MW 94 boiling point bp 182 C 3596 F has a boiling point more than 70 degrees higher than that of toluene C 6 H 5 CH 3. 50 g of urea NH 2 CONH 2 is dissolved in 850 g of water. Solved Examples Example 1. Aromatic Chloroform Odor Vapor pressure. -1958 C -3204 F Boiling point of liquid helium.
 Source: researchgate.net
Source: researchgate.net
This decrease will affect the time it takes to cook anything in water to the extent that any food that requires five minutes to prepare at. 56 C 1328 F Boiling point of alcohol. Formerly used as an inhaled anesthetic during surgery the primary use of chloroform today is in industry where it is used as a solvent and in the production of the refrigerant freon. In order for a liquid to boil its vapor pressure needs to exceed ambient pressure which is harder to achieve once you add a nonvolatile component. Not determined Vapor density.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title chloroform boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.